
Figure 1. An adult annual bluegrass weevil. Photos by Ben McGraw
The annual bluegrass weevil (ABW), Listronotus maculicollis (Kirby), is considered the most devastating pest of short-mown golf course turf (tees, fairways, collars, putting greens) in the northeastern and Mid-Atlantic United States and eastern provinces of Canada (5). Larvae are stem borers until their third instar, though their feeding may not produce noticeable damage. Most damage comes from fourth- and fifth-instar larvae feeding externally on the crown of the plant, especially within turfgrass stands with a high percentage of annual bluegrass (Poa annua L.) (4). A single larva may kill up to a dozen plants through external crown feeding and at high larval densities (> 40/square foot), with damage appearing as large patches of yellow and brown turf (1).
Most turf managers attempt to minimize larval damage by applying insecticides to adults newly emerged from overwintering habitats in spring. The emergence of weevils from overwintering sites occurs over several weeks, beginning as early as March and ending in late April to early May in most areas surrounding the epicenter of the weevil’s distribution (metropolitan New York City area). The beginning and duration of their emergence will vary intra- and inter-seasonally with temperature, which hinders the ability of turfgrass managers to effectively target populations with insecticides.
Consequently, many turfgrass managers report making multiple insecticide applications, which has led to the development of pyrethroid-resistant populations (8) on an estimated 20% of golf courses in eastern North America (7).

Right: Figure 2. Copulating annual bluegrass weevils.
Upon arrival at the short-mown turf areas, adult ABW will feed, mate and oviposit (lay eggs) into the stems of grass plants, though the timing of these events remains unstudied (Figure 2). Occasional observations of leaf notching on short-mown stands indicates that adult feeding commences soon after emergence. However, the period in which females are mated and begin to oviposit is unclear, although one unpublished study suggests that only a small percentage of females is inseminated prior to overwintering (9). Understanding when these events occur is critical to ABW management, as the only effective insecticides to control adults are active ingredients with short residual activity (for example, pyrethroids, organophosphates) that require precise timing (2).
The objectives of this study were to 1) describe the reproductive phenology of emerging ABW populations, including their sexual maturity, mating and reproductive longevity; 2) determine the incidence of adult feeding after emergence; and 3) determine adult reproductive longevity, female fecundity and time sequence of egg deposition in controlled (caged) experiments. By better understanding ABW reproductive phenology, we will be able to employ more accurate and efficient management strategies that increase the longevity of chemical controls by delaying the development of resistance.
Materials and methods
Insect collections
Adult ABW populations were sampled weekly as they emerged from overwintering sites through the duration of the overwintering adult oviposition period (March-May) from 2011 through 2013 on The College Golf Course at Delhi (Delhi, N.Y.). Six locations (three putting green collars, three tee boxes) were chosen based on past ABW damage. Each study site was near (15 to 50 feet; 4.6 to 15.2 meters) or immediately adjacent to (< 15 feet) a potential adult overwintering site (for example, tall grasses, base of tree, leaf litter, wood line).
Adults were collected with a leaf blower/vacuum (Echo ES-255) fit with a mesh basket to capture weevils before they entered the engine (6). Five samples were taken sequentially from each collar or tee box by vacuuming each sample unit for 15 seconds (~30 to 40/square foot [0.09 square meter]). Adults were frozen within one hour of field collection and dissected within 12 hours.
The sampling period began before traditional plant phenological indicators (Forsythia species) would indicate adult movement from overwintering sites (< 100 growing degree days [GDD]; base temperature = 50 F; March 1 start date) on April 22, 2011; March 18, 2012; and April 23, 2013. Observations ended after a significant proportion of females (>90%) in the population displayed spent reproductive systems (that is, no oocytes in the vitellarium, no eggs in the calyx) and/or the adult densities became too low (< 0.5 adult average per site) on July 1, 2011; Aug. 6, 2012; and July 16, 2013.
Insect dissections
Adults were dissected and the presence of new food in the gut as well as relative status of male and female reproductive organs were visually assessed under a microscope. The incidence of recent feeding was readily apparent following the removal of the elytra.
Male and female reproductive system development was categorized (1, 11, 12) as small, medium or large (reproductively mature) based on size and width of reproductive organs (Figures 3, 4, below). Mating status was determined as the presence of sperm in the spermatheca. The number of eggs in the calyces was recorded in females with large reproductive systems.
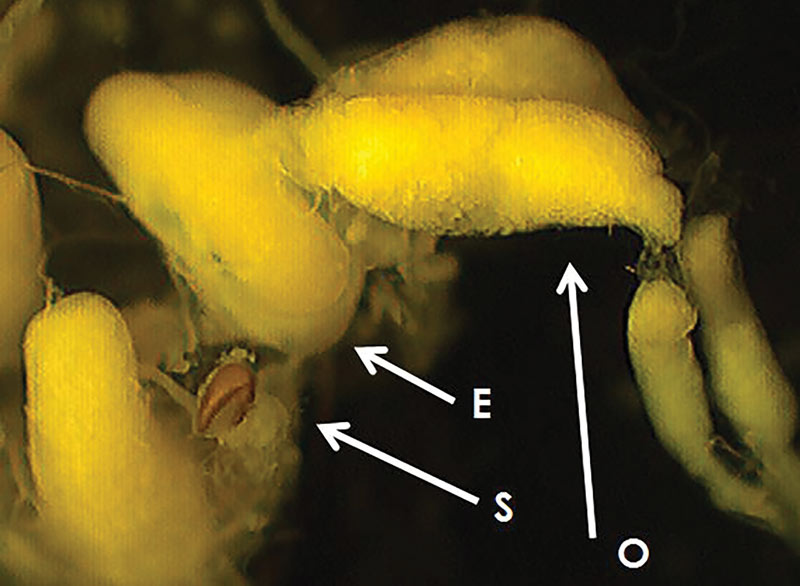
Figure 3. Reproductive system of a mature female annual bluegrass weevil. S = spermatheca; E = egg; O = ovariole.
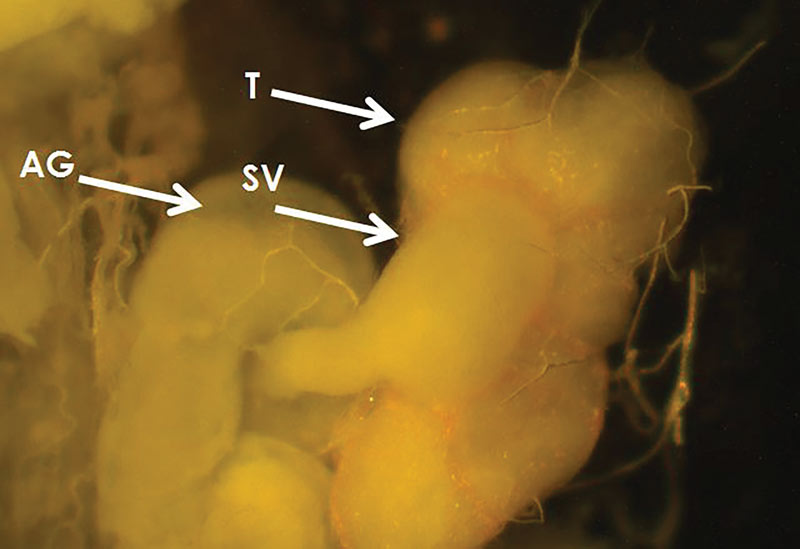
Figure 4. Reproductive system of a mature male annual bluegrass weevil. SV = seminal vesicle; T = testis; AG = accessory gland.
Female fecundity and ovipositional patterns
Individual pairs of males and females were caged on Poa annua plugs (0.5-inch [1.27-centimeter] height of cut) during the period following overwintering emergence (2012-2013) and observed weekly until they expired. Forty-five cohorts, consisting of one female and one male, were added to cages coinciding with the start of weevil emergence (79 GDD, April 7, 2012; 88 GDD, May 2, 2013). Cages were 50-milliliter (1.7-fluid-ounce) conical test tubes with mesh netting to prevent weevils from escaping (Figure 5, below). Turf plugs (1.2-inch diameter [3-centimeter diameter]) were taken from the nursery where the trial was held and placed in each cage to provide mating pairs with a food source and an oviposition substrate. The conical tubes holding the weevils and Poa annua were inserted into the ground until the top of the turf plug was flush with the shoots of the surrounding turf.
Adults were extracted by hand or flushed with lukewarm water once a week. Survivors were moved to fresh Poa annua cores. Each tiller from the old core was examined for eggs (or larvae) by peeling back the leaf blade from the stem and examining under a microscope (Figure 6, below). Each mating pair was tracked until they expired, and the experiment concluded when no females were left (1229 GDD, July 16, 2012; 1280 GDD, July 24, 2013). Degree-day accumulations were recorded on-site to determine the correlation between degree-day accumulations and egg laying. Oviposition, as a function of weekly GDD accumulations, was assessed by fitting curves to the number of eggs deposited over the course of the experiment to develop a predictive oviposition model based on degree-day accumulations.

Figure 5. Annual bluegrass weevil mating cohorts.

Figure 6. Two recently oviposited annual bluegrass weevil eggs (yellow) can be seen through the leaf sheath on this Poa annua tiller.
Results
Adult emergence and feeding
Adult emergence differed substantially by calendar date among the three seasons. Little snowfall was observed in the winter of 2011-2012, and first adult emergence was observed five weeks earlier than in 2011 or 2013. Adult emergence relative to degree-day accumulations was a better predictor than calendar date, though still variable between years of the study (24 and 109 GDD; Figure 7, below). Two peaks in adult density were consistently observed in all years. The first (and most dense) peak occurred between 122 and 139 GDDs despite differing by as much as three weeks between 2012 and 2013. In 2011, peak density occurred at 172 GDD.
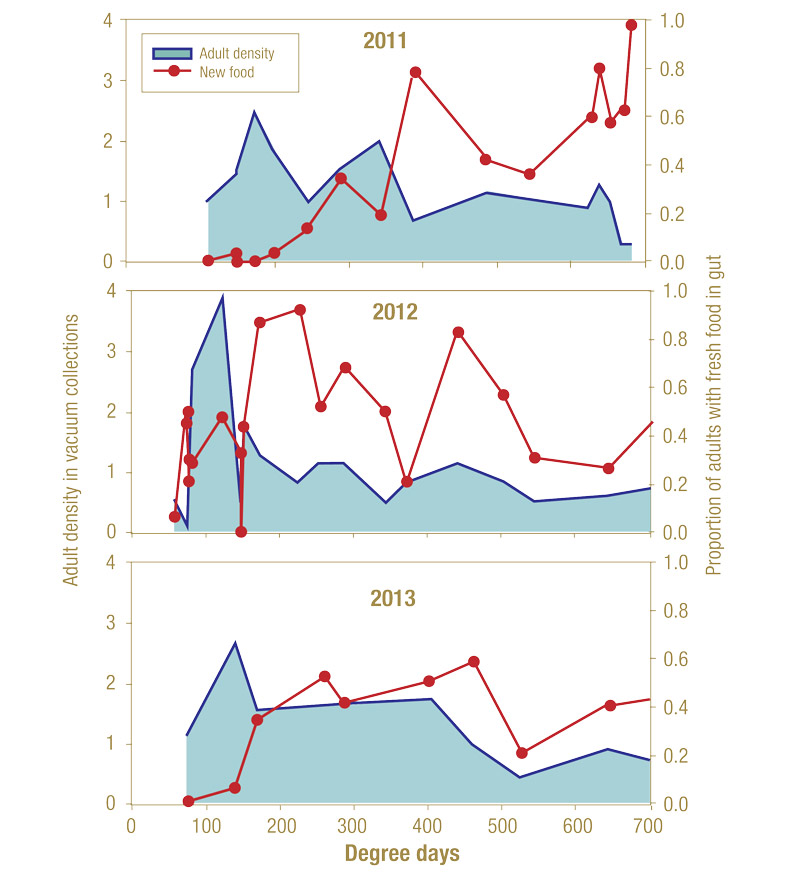
Figure 7. Seasonal phenology of feeding in populations of adult annual bluegrass weevils vacuum-collected from greens and tees at The College Golf Course at Delhi (Delhi, N.Y.; 2011-2013). Growing degree day monitoring began on March 1 (base temperature = 50 F). Growing degree days were calculated using the integral sine method using an on-site weather station. Adult density is calculated as the average number of adults collected in 15-second vacuum samples.
A low percentage of overwintering weevils collected from playing surfaces was observed to have fed immediately before capture on the short-mown areas. In 2011 and 2013, none of the colonizing adults fed before capture on greens or tees, and only a small percentage (<10%) of the first arrivers in 2012 had new food in their guts. Adult feeding steadily increased in the weeks following emergence, with peaks of adults with new food in guts occurring after the second adult density peak in all years.
Reproductive phenology
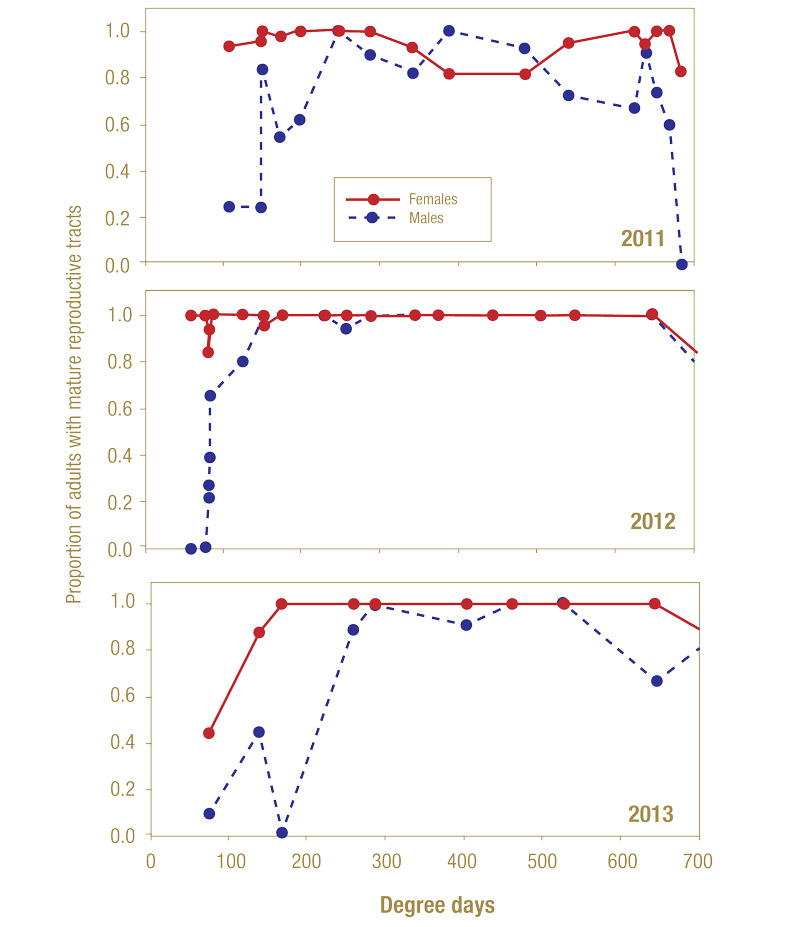
Figure 8. Relative proportions of annual bluegrass weevil adults with mature reproductive systems (Delhi, N.Y.; 2011-2013).
Reproductive development differed between males and females in each year of the study (Figure 8). In 2011 and 2012, the vast majority (>95%) of females collected on short-mown areas during the earliest sampling dates had mature (large) reproductive systems. In all years, all females captured by the third week possessed large, well-developed reproductive systems. Few of the initial captures of males (less than 30% of the population) were observed with mature (large) reproductive systems. Throughout the following weeks, the proportion of mature males steadily increased, as did feeding. In 2011 and 2013, all males were mature after 200 GDD, with the 2012 population maturing after 280 GDD.
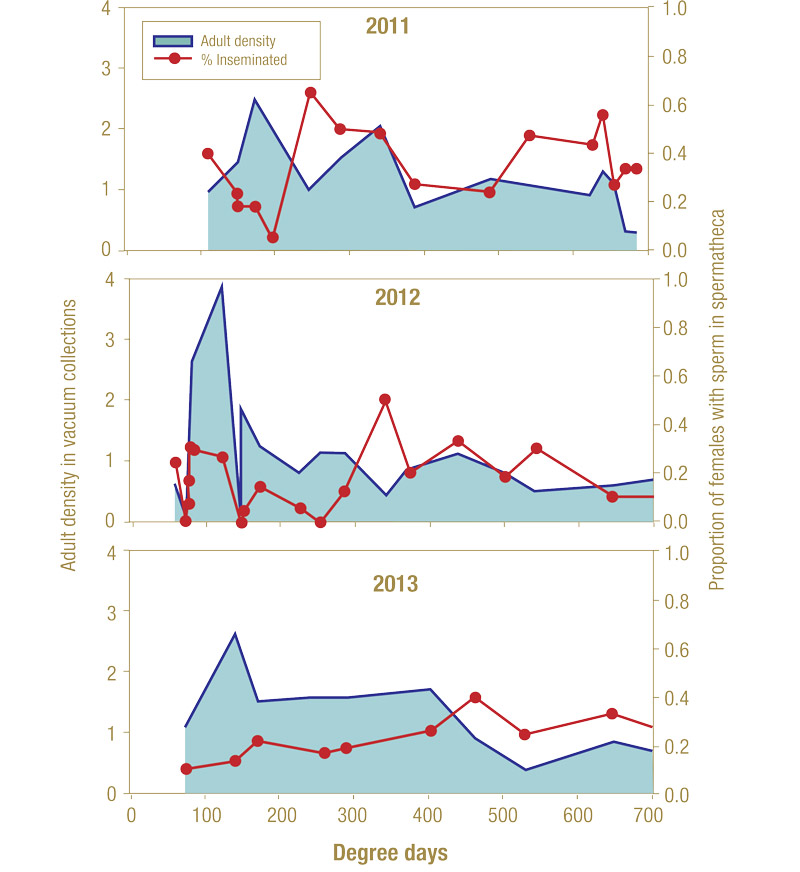
Figure 9. Proportion of annual bluegrass weevil females with sperm present in the spermatheca (mated) and the average number of eggs per calyx (Delhi, N.Y., 2011-2013).
A low to moderate proportion of the female population was found to have sperm in their spermatheca at any time during the study (Figure 9). The highest percentages of inseminated females (40% to 60%) in a season were detected after the second adult density peak (~250 to 460 GDD) in each year of the study. Females were found with eggs in their calyx when they arrived at oviposition sites. Populations did not display a defined peak or synchronicity in the development of eggs, and, like mating, the average number of eggs did not show any consistent trends between seasons.
Female fecundity and ovipositional patterns
In 2012, adults survived up to 15 weeks in confinement, with oviposition observed as late as 14 weeks. Adult survival steadily declined over the observation period, averaging 8.49 ± 0.68 weeks (median = 9) for females and 8.27 ± 0.69 (median = 8) for males. In 2013, high adult mortality, possibly caused by cold conditions during the start of the experiment, was observed in the first week and greatly reduced average post-emergence survival times of both males (4.84 ± 0.65; median = 4 weeks) and females (5.58 ± 0.67 weeks; median = 4 weeks). These differences led to differences in duration of the female’s oviposition period between years of the study, though oviposition followed a skewed normal distribution, with bias for the beginning weeks of confinement in both years (Figure 10, below).
Average total fecundity ranged between 62.9 (±7.05 SE) eggs, as observed in 2013, and 92.6 (±12.7 SE) eggs, as observed in 2012. Fecundity was greatest in the first five (2013) to eight weeks (2012) of the experiments, after which there was a dramatic drop and leveling off in oviposition. At peak egg-laying, females produced approximately three eggs per day.
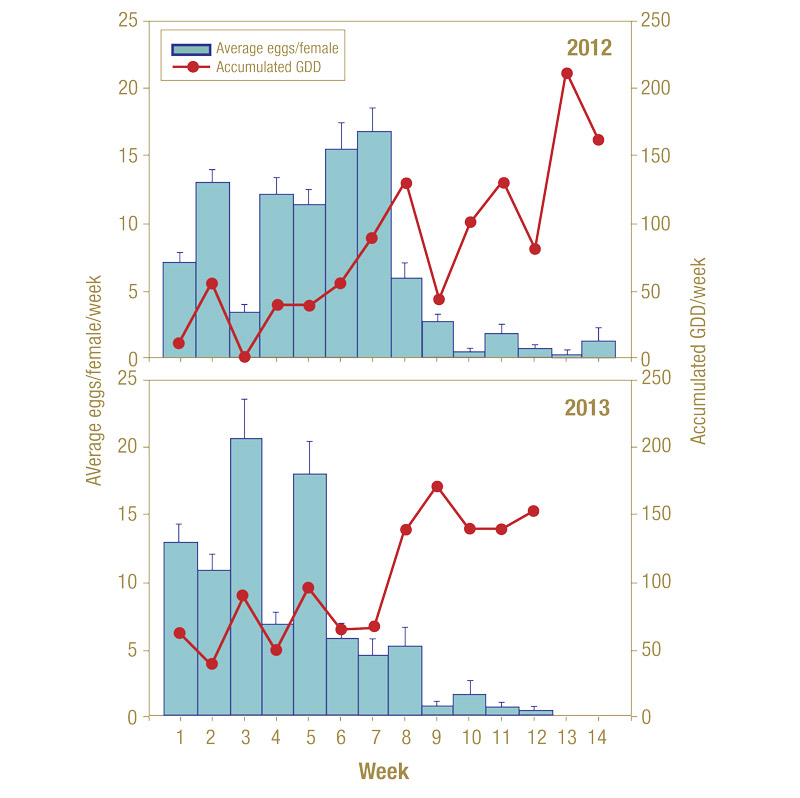
Figure 10. Average number of eggs per annual bluegrass weevil female per week in mating enclosures (1 female + 1 male) and accumulated growing degree days during the week.
Strong correlations were found between degree-day accumulations and average eggs laid per female over the periods before the decrease in egg production (R2 = 0.96 and 0.81 for 2012 and 2013, respectively), and pooling data sets also resulted in a strong correlation over the first five weeks of egg laying (R2 = 0.83). Oviposition models provided correlations (R2 > 0.81) between oviposition in the first five weeks of confinement and degree-day accumulations.
Discussion
Observations of natural populations of ABW conducted over several years on golf courses indicate that most adults do not feed between emerging from overwintering sites and arriving on short-mown playing surfaces in spring. These findings suggest that control programs that are designed to intercept migrating adults in spring with systemic insecticides that require ingestion are unlikely to provide control. This study confirmed earlier reports that a low percentage of females are mated prior to emergence (9). These findings would suggest that turfgrass managers who wish to control adults with short-residual, contact insecticides would benefit from waiting until peak adult emergence from overwintering sites to allow for maximum exposure.
Editor’s note: What effect do different vegetation management practices in golf course natural areas have on weevil winter mortality? Read the research.
Our study suggests that calendar date of emergence of overwintering adults can vary greatly and that estimating population emergence based on growing-degree-day accumulations provided a more reliable estimate of activity. However, springtime temperatures fluctuated greatly in the period between the first adult captures and adult density peaks in all years of the study. Therefore, turfgrass managers who apply insecticides based solely on visual observations of adults would have a greater probability of having to reapply, especially given that the adulticides labeled for use against ABW have short residual activity.
Peak adult densities were more consistent than emergence and occurred in a discrete temperature accumulation range in each year of the study (122 to 172 GDD). These values, though specific to this study, have been observed to be reliable estimates for peak emergence of adults in areas outside this region in the period since the study was conducted. These findings suggest that golf course superintendents may benefit from using on-site weather stations or growing-degree-day data loggers to predict population peaks, rather than having to rely on unrelated plant phenological indicators such as Forsythia species and flowering dogwood.
Although our study demonstrates that insecticides applied during the initial adult migration from overwintering sites might provide little benefit to ABW population control, it does not diminish the merit of adulticide applications. ABW populations have multiple generations per year (multivoltine) in all but the most northern areas of the weevil’s distribution (10), and control during the second or third generation is often more challenging than earlier in the season because of the presence of stages that are not susceptible to chemical controls (for example, eggs, stem-boring larvae and pupae) (3).
The period when adults emerge from overwintering sites represents the only time of the year when one stage is present and provides the potential for control with one insecticide. A battery of insecticide applications is often needed to reduce the potential for larval damage if adults are left uncontrolled.
Female ABW were found to be much more fecund than what had been previously reported in laboratory studies (1), which may be due, in part, to conducting the experiment in a more natural setting. More importantly, we observed that females could oviposit over a 14-week period in captivity, with the greatest number of eggs deposited over the first five- to eight-week period. Therefore, untreated populations are likely to have asynchronous populations from the beginning of the season, which, in turn, would make control with larvicides more difficult (3).
We observed that timing of reproductive development differed between the sexes. Females possessed mature reproductive systems and were capable of ovipositing early in the spring. However, few are inseminated before winter (as demonstrated by the incidence of females with sperm in the spermatheca) and thus would require mature males to produce fertilized eggs. Early arriving males were not shown to be reproductively mature and required several weeks before a large proportion of the population matured. The incidence of feeding followed a similar trend, but, to date, it is still unclear whether the two events are correlated.
There were no clear trends in the number of eggs per calyx or the proportion of inseminated females, even after most males in the population had matured. This may indicate that mating occurs throughout a female’s lifetime and that egg production is continuous, and may explain what causes the overlapping, asynchronous developmental stages in summer generations that are difficult to forecast and control with conventional insecticides.
This is the first study of its kind to describe the ovipositional behaviors and physiological development of overwintered ABW populations. Female ABW oviposit over many weeks, which may explain why poorly timed adulticides, such as early-emergence applications with combination products, often lead to rapid development of asynchronous larval populations during the first and subsequent generations. We recommend that adulticides not be applied until populations have reached peak densities, as males are unlikely to be mature and few females are inseminated before this period.
The results from this study will help in developing precision management programs, eliminate unnecessary insecticide applications (and associated costs), slow the development of insecticide resistance, and ultimately reduce the probability for environmental contamination.
Acknowledgments
The authors would like to thank the New York State Turfgrass Association for its support through the New York State Community IPM program grant. Special thanks to Thomas Holdrege for technical assistance, and to Thomas Kaufman, golf course superintendent at The College Golf Course at Delhi (N.Y.), for use of the golf course.
The research says ...
- Superintendents typically attempt to control the annual bluegrass weevil by applying insecticides in spring when adults are newly emerged from overwintering habitats.
- Because most ABW adults do not feed between emergence from overwintering sites and arrival on short-mown golf course surfaces in spring, systemic insecticide applications are unlikely to be effective against adults.
- Superintendents may benefit from using on-site weather stations or growing-degree-day data loggers to predict population peaks.
- We recommend that adulticides not be applied until populations have reached peak densities, as males are unlikely to be mature and few females are inseminated before this period.
Literature cited
- Cameron, R.S., and N.E. Johnson. 1971. Chemical control of the “annual bluegrass weevil,” Hyperodes sp. nr. anthracinus. Journal of Economic Entomology 64(3):689-693 (https//:doi.org/10.1093/jee/64.3.689).
- Koppenhöfer, A.M., S.R. Alm, R.A. Cowles, B.A. McGraw, S. Swier and P.J. Vittum. 2012. Controlling annual bluegrass weevil: optimal timing and rates. Golf Course Management 80:98-104.
- Koppenhöfer, A.M., B.A. McGraw, O.S. Kostromytska and S. Wu. 2019. Variable effect of larval stage on the efficacy of insecticides against Listronotus maculicollis (Coleoptera: Curculionidae) populations with different levels of pyrethroid resistance. Crop Protection 125:104888.
- Kostromytska, O.S., and A.M. Koppenhöfer. 2014. Ovipositional preferences and larval survival of annual bluegrass weevil, Listronotus maculicollis, on Poa annua and selected bentgrasses (Agrostis spp.). Entomologia Experimentalis et Applicata 152(2):108-119 (https//:doi.org/101111/eea.12204).
- McGraw, B.A., and A.M. Koppenhöfer. 2007. Biology and management of the annual bluegrass weevil, Listronotus maculicollis (Coleoptera: Curculionidae). Pages 335-350. In: M. Pessarakli, ed. Handbook of Turfgrass Management and Physiology. CRC Press, Boca Raton, Fla. (https//:doi.org/10.1201/9781420006483).
- McGraw, B.A., and A.M. Koppenhöfer. 2009. Development of binomial sequential sampling plans for forecasting Listronotus maculicollis (Coleoptera: Curculionidae) larvae based on the relationship to adult counts and turfgrass damage. Journal of Economic Entomology, 102(3):1325-1335 (https://doi.org/10.1603/029.102.0360).
- McGraw, B.A., and A.M. Koppenhöfer. 2017. A survey of regional trends in annual bluegrass weevil (Coleoptera: Curculionidae) management on golf courses in eastern North America. Journal of Integrated Pest Management 8(1):2 (https//:doi.org/10.1093/jipm/pmw014).
- Ramoutar, D., S.R. Alm and R.S. Cowles. 2009. Pyrethroid resistance in populations of Listronotus maculicollis (Coleoptera: Curculionidae) from southern New England golf courses. Journal of Economic Entomology 102(1):388-392 (https//:doi.org/10.1603/029.102.0150).
- Rothwell, N.L. 2003. Investigation into Listronotus maculicollis (Coleoptera: Curculionidae), a pest of highly maintained turfgrass. Ph.D. dissertation. University of Massachusetts, Amherst, Mass.
- Simard, L., J. Brodeur and J. Dionne. 2007. Distribution, abundance, and seasonal ecology of Listronotus maculicollis (Coleoptera: Curculionidae) on golf courses in Quebec, Canada. Journal of Economic Entomology 100(4):1344-1352.
- Snow, S.J. 1928. Effect of ovulation upon seasonal history in the alfalfa weevil. Journal of Economic Entomology 21(5):752-761 (https//:doi.org/10.1093/jee/21.5.752).
- Vittum, P.J. 1980. The biology and ecology of the annual bluegrass weevil, Hyperodes sp. near anthracinus (Dietz) (Coleoptera: Curculionidae). Ph.D. dissertation. Cornell University, Ithaca, N.Y.
Benjamin A. McGraw is a professor in the Department of Plant Science and Garrett Y. Price and Audrey Simard are graduate students in the Department of Entomology at Penn State University, University Park, Pa. Patricia J. Vittum is professor emeritus in the Stockbridge School of Agriculture at the University of Massachusetts, Amherst, Mass.