
Black cutworm stages, from left: third, fourth, fifth and sixth larval stage, prepupa, pupa. Photos by Lemma Ebssa
Black cutworm (BCW; Agrotis ipsilon) larvae are one of the most widespread and perennial pests of golf course tee boxes and putting greens (4). Depending on latitude, the BCW has two to six generations per year. The first two instars feed on the leaf
blades, causing no significant damage. The third through final instars hide in burrows in the thatch or soil by day. At night, they feed on stems and foliage, creating sunken areas, pockmarks and dead patches that make the turf unattractive and disrupt
the uniformity and smoothness of the putting surface. Predation by birds on the larvae can cause further disruption of the turf surface (4). Pupation occurs in the larval burrows.
Turf areas affected by BCW are commonly treated with broad-spectrum synthetic insecticides several times per year. Biological and microbial control agents such as entomopathogenic nematodes (EPNs) may offer an alternative for BCW management that is safer
and without significant negative effects on natural enemies of arthropod pests. EPNs are microscopic, insect-parasitic roundworms that possess an infective juvenile stage common in the soils of many ecosystems.
Various EPN species have been shown to effectively control numerous insect pests in a multitude of commodities including turfgrass (2). With BCW, laboratory studies in pots with grass including seven EPN species (Heterorhabditis bacteriophora, H. megidis,
H. indica, Steinernema carpocapsae, S. riobrave, S. feltiae and S. kraussei) and BCW stages from the third larval stage through the pupa found that at constant 77 F (25 C), S. carpocapsae tended to cause the highest larval mortality, followed by H.
bacteriophora, H. megidis and S. riobrave (1). However, at constant 66 F (19 C), S. feltiae was one of the top performers. Moreover, fourth and/or fifth instar larvae were the most susceptible stage to most EPN species, and pupae were the least susceptible.
Our objective was to compare the field efficacy of several commercial EPN species/strains for BCW control.
Because EPNs are sensitive to environmental extremes, their successful use may require some modifications compared to synthetic insecticide use (2). We therefore explored whether EPN efficacy and persistence could be increased by splitting applications
and by syringing.
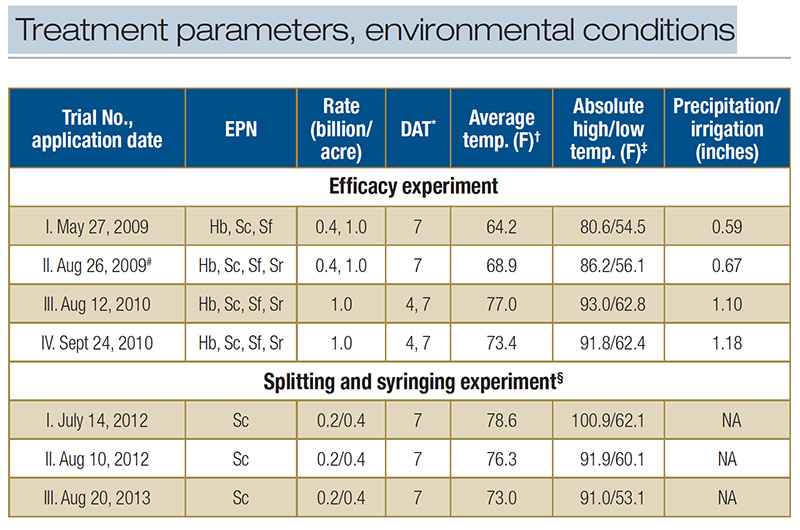
Table 1. Treatment parameters and environmental conditions during microplot field trials testing the entomopathogenic nematodes (EPN) Heterorhabditis bacteriophora (Hb), Steinernema carpocapsae (Sc), S. feltiae (Sf) and S. riobrave (Sr) for BCW control in a creeping bentgrass field maintained under golf course fairway conditions. *Larval survival was assessed 7 days or 4 and 7 days after nematode application (DAT). ?Temperature averaged across entire trial period. ‡Highest and lowest temperature during entire trial period. #Sc and Sr were applied at both rates, Hb and Sf at high rate only. §In this experiment, some treatments were split into two applications applied at 0 and 4 DAT. Plots received no overhead irrigation and there was no rainfall. Plots that received syringing received 0.08 inches irrigation twice per day for a total of 1.1 inches
Materials and methods
Insects and nematodes
BCW larvae were reared from eggs obtained from Benzon Research Inc. (Carlisle, Pa.) on a BCW diet. In the efficacy study, we released fourth-instar larvae; in the splitting/syringing study, we released a mixture of third-, fourth- and fifth-instar larvae
to simulate more natural conditions. Fresh commercial products based on the EPN species S. carpocapsae (Product name: Millenium), S. feltiae (Nemasys), S. riobrave (BioVector) and H. bacteriophora (Nemasys G) were obtained from Becker Underwood Inc.
(Ames, Iowa), kept at 46 F (8 C) and used for experiments within one month of delivery. These species/strains had shown high virulence against BCW larvae in previous studies (1). For the efficacy experiments, EPN products were suspended in tap water
and the concentrations of the suspensions determined and adjusted before application. For the splitting/syringing experiments, S. carpocapsae was reared in wax moth (Galleria mellonella) larvae following standard procedure to standardize quality for
experiments.
Nematode efficacy
Methods
Experiments were conducted at the Rutgers University Horticultural Farm II (North Brunswick, N.J.) in a 1-to-2-year-old creeping bentgrass (Agrostis stolonifera) field mowed three to four times per week at 0.375 inches (9.5 millimeters). The field was
not treated with insecticide and was irrigated and fertilized as needed to keep the turf healthy. Experimental arenas consisted of turf plots (1 foot × 1 foot; 30.5 centimeters × 30.5 centimeters) surrounded by a 5-inch-high (13-centimeter-high)
plastic barrier made from garden edging material (Emerald Edge, Easy Gardener, Waco, Texas) pushed into the ground to a depth of 2 inches (5 centimeters). The inner sides of the barrier were painted with Fluon (BioQuip Inc., Rancho Dominguez, Calif.)
to contain released larvae. Plots were arranged in a randomized complete block design with 10 blocks. Adjacent plots were separated by a 4-inch (10-centimeter) buffer.
After the release of 10 fourth-instar BCW per plot, the plots were covered with steel mesh with openings of 0.5 inches × 0.5 inches (1.3 centimeters × 1.3 centimeters) to prevent bird predation. After 24 hours, treatments were applied in 0.3
fluid ounces (10 milliliter) tap water per plot using spray bottles. Untreated control plots received water only.
Nematodes were watered in with 6.8 fluid ounces (200 milliliters) water per plot (equivalent to 0.08 inches [2 millimeters] irrigation water).
Larval survival was assessed four or seven days after application using a soap flush (6.8 fluid ounces of 0.2% concentrated lemon-scented dish detergent in water per plot) to irritate surviving larvae to the turf surface.
Four experiments were conducted. For applications, evaluation intervals, EPN species and rates, and environmental conditions, see Table 1.
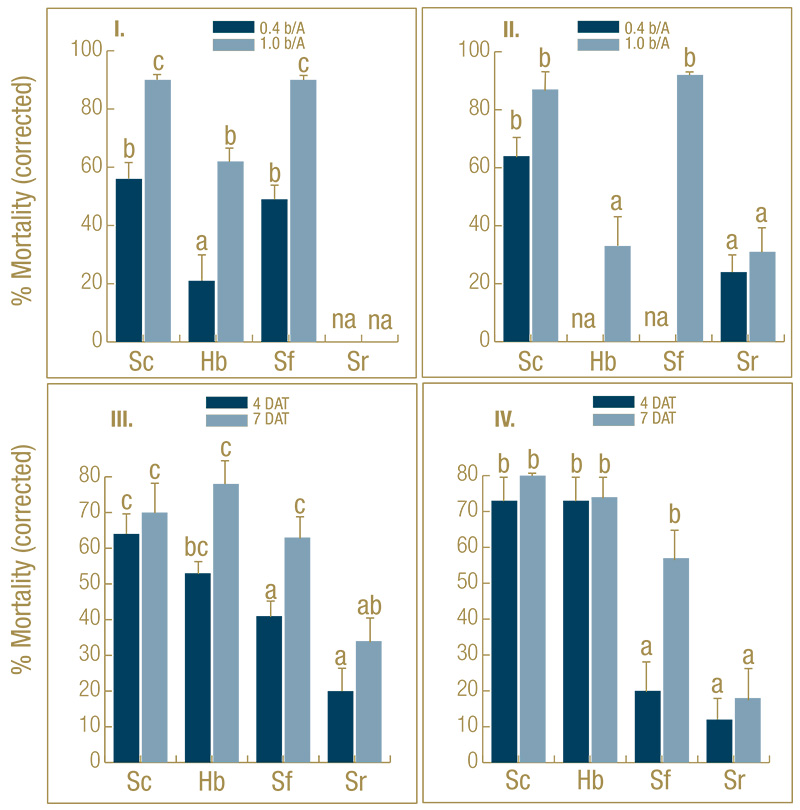
Figure 1. Effect of EPN species (Sc=Steinernema carpocapsae, Sf=S. feltiae, Sr=S. riobrave, Hb=Heterorhabditis bacteriophora) on percentage control (mean ± SEM) of black cutworm larvae in microplots infested with fourth-instar larvae under golf course fairway conditions. In experiments I and II, the nematodes were compared at two rates (0.4 and 1.0 billion nematodes/acre) at 7 days after treatment (DAT). In experiments III and IV, the nematodes were compared at the high rate only but after 4 and 7 DAT. na=treatment was not applied. Means with the same letter do not differ significantly within experiment (Tukey’s HSD, α = 0.05).
Results
In the untreated control plots, 70% to 85% of the larvae were recovered. Control-corrected larval mortality was significantly affected by EPN species and rate and exposure length. Across the four experiments, S. carpocapsae was the most effective, most
consistent and fastest-killing species (Figure 1), whereas S. riobrave was ineffective. At the high rate (1 billion nematodes/acre) after seven days, S. carpocapsae (control averaged across the four experiments: 82%, range 70%-90%) did not cause significantly
higher mortality than S. feltiae (76%, range 57%-92%) but was more consistent. Both species were significantly more effective than H. bacteriophora (62%) in two out of four experiments. S. carpocapsae also caused higher mortality (60%) at the low
rate (0.4 billion nematodes/acre) than S. feltiae (49%) and H. bacteriophora (21%). At 4 DAT, S. carpocapsae (70%) caused significantly higher mortality than S. feltiae (31%) but not significantly higher than H. bacteriophora (63%).
Black cutworm microplots.
Split application and syringing on S. carpocapsae efficacy
Methods
Three experiments were conducted and evaluated using the same general methodology and field as for the efficacy experiments. The field had received enough rainfall or overhead irrigation before nematode application to provide soil moisture near saturation
at the beginning of the experiments. During the experiments, the plots received no overhead irrigation, and, if rainfall was imminent, were covered with a plastic tarp until any rainfall had stopped. Steinernema carpocapsae was applied at low rates
to allow for the expression of treatment effects. Rates were applied once at the beginning of the experiment or in a split application with half of the total applied at the beginning of the experiment and the rest three days later. Control plots received
water only. Untreated plots and each S. carpocapsae treatment were further randomly assigned to receive syringing or not. Syringing consisted of 0.08 inches (2 millimeters) of irrigation applied with a watering can at 12 p.m. and 3 p.m. (EDT) on each
day.
Hence, treatments consisted of: (1, 2) no nematodes with or without syringing; (3, 4) S. carpocapsae at 0.2 billion/acre applied at zero DAT with or without syringing; (5, 6) S. carpocapsae at 0.4 billion/acre applied at zero DAT with or without syringing;
(7) S. carpocapsae at 0.1 billion/acre applied at zero and 3 DAT without syringing; and (8) S. carpocapsae at 0.2 billion/acre applied at zero and 3 DAT without syringing. In each experiment, there were 10 replicates per treatment and untreated control
arranged in a randomized complete block design. Larval mortality was evaluated seven days after the first application. Experimental periods and weather conditions are listed in Table 1.
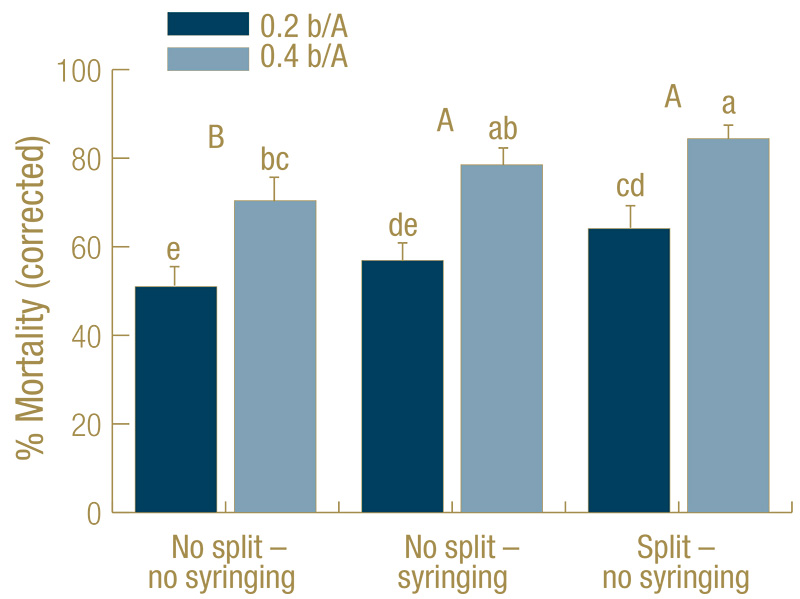
Figure 2. Mortality (percent control-corrected) of Agrotis ipsilon larvae (mix of third through fifth instar) at 7 days after treatment (DAT) caused by the entomopathogenic nematode Steinernema carpocapsae applied at 0.2 or 0.4 billion nematodes per acre under golf course fairway conditions. The full rate was applied at 0 DAT (no split) or half of the rate applied at the beginning, the other half at 3 DAT (split). Treatments received no irrigation during the experiment (no syringing) or received 2 millimeters overhead irrigation twice each day (syringing). There was no rainfall during the experiments. Data are combined from three experiments (n=30). Columns with the same lower-case letter do not differ significantly; capital letters indicate significant differences when the two nematode rates were combined for analysis (Tukey’s HSD, α = 0.05).
Results
In all experiments, all treatments including S. carpocapsae caused significant mortality, but the untreated controls with (average 21% mortality) or without (22%) syringing never differed from each other significantly. Hence, the data were control-corrected
with the average of the two untreated controls in each experiment and combined across experiments. For the control-corrected data, mortality across both S. carpocapsae rates was significantly higher for split applications without syringing (74%) and
the single application with syringing (68%) than for single applications without syringing (61%). The higher rate (78%) was more effective than the lower rate (57%) (Figure 2).
Conclusions
Our findings show that commercial EPN formulations can control BCW larvae on golf course turf. Among the four species tested, S. carpocapsae was the best-performing species due to a combination of high control rates, most consistent results and higher
speed of kill. Efficacy of S. feltiae and H. bacteriophora was often similar to that of S. carpocapsae but overall lower and less consistent. Steinernema riobrave was consistently ineffective. S. carpocapsae provided 80%-90% control of BCW under average
late-spring and summer temperatures despite a high larval density. Furthermore, it controlled the larvae quickly, with 70% control within 4 DAT.
Even under warm conditions (mean of 77 F: Trial III), S. carpocapsae provided 70% control. However, this control rate would not suffice to meet the high control standards on a golf course green. Thus, when possible, EPN should be applied during days with
lower day temperatures and smaller temperature fluctuations between day and night and between consecutive days after application. But this may not always be possible. Instead, S. carpocapsae efficacy could be improved in general and made more dependable
under hot conditions through syringing and split applications as shown in the second part of our study.
Syringing improved S. carpocapsae efficacy by on average 8% and also significantly reduced control variability (mean 78%, range 75%-87%) at the low rate of 0.4 billion nematodes per acre. The beneficial effect of syringing on S. carpocapsae performance
can in part be ascribed to the improved persistence of the infective juveniles in the syringing regime (3). Splitting applications into two applications at half rate applied three days apart proved to be more effective than syringing. This improved
efficacy by 13% on average while significantly reducing control variability (mean 84%, range 80%-92%) at 0.4 billion nematodes per acre.
What still needs to be tested is how effective a combination of S. carpocapsae with both syringing and split applications would be. However, we see no reason why their combination should not combine the benefits of both methods, resulting in highly effective
and reliable BCW control. Since increasing the S. carpocapsae rate from 0.4 billion per acre to the typically recommended field rate of 1 billion per acre generally resulted in 24%-33% higher control rates, use of the recommended rate combined with
split applications and syringing would likely result in consistent control of 90% or higher and could make this approach competitive with synthetic insecticide use.

Black cutworm fifth instar. Photo by Albrecht Koppenhöfer
The Research Says
- S. carpocapsae was the best-performing EPN species for BCW control due to a combination of high control rates, most consistent results and higher speed of kill.
- A commercial S. carpocapsae product provided 80%-90% BCW control under average late spring and summer conditions but only 70% under hot summer conditions.
- Syringing improved S. carpocapsae performance by 8% and also reduced control variability.
- Splitting applications into two applications applied three days apart improved S. carpocapsae performance by 13% and also reduced control variability.
- Splitting applications and syringing should make S. carpocapsae applications more effective and should reduce the negative effects of hot summer conditions.
Funding
This research was supported by the Rutgers Center for Turfgrass Science and the USDA National Institute of Food and Agriculture Hatch Multistate project 0206130 through the New Jersey Agricultural Experiment Station (Hatch Multistate project NJ08295).
Acknowledgments
The authors thank the maintenance staff of Rutgers Horticultural Farm No. 2. This article was based on two published papers, “Efficacy and persistence of entomopathogenic nematodes for black cutworm control in turfgrass” by L. Ebssa and A.M.
Koppenhöfer in 2011 in Biocontrol Science and Technology (21:779-796) and “Species combinations, split applications, and syringing to optimize the efficacy of entomopathogenic nematodes against Agrotis ipsilon (Lepidoptera: Noctuidae) larvae
in turfgrass” by A.M. Koppenhöfer, O.S. Kostromytska and L. Ebssa in 2022 in Crop Protection (155:1-8).
Literature cited
- Ebssa, L., and A.M. Koppenhöfer. 2012. Entomopathogenic nematodes for the management of Agrotis ipsilon: effect of instar, nematode species and nematode production method. Pest Management Science 68:947–957 (https://doi.org/10.1002/ps.3259).
- Koppenhöfer, A.M., D.I. Shapiro-Ilan and I. Hiltpold. 2020. Advances in the use of entomopathogenic nematode biopesticides in suppressing crop insect pests. Pages 1-38. In: Biopesticides for Sustainable Agriculture. N. Birch, T. Glare (eds.).
Burleigh Dodds Science Publishing, Cambridge, U.K.
- Koppenhöfer, A.M., O.S. Kostromytska and L. Ebssa. 2022. Species combinations, split applications, and syringing to optimize the efficacy of entomopathogenic nematodes against Agrotis ipsilon (Lepidoptera: Noctuidae) larvae in turfgrass. Crop
Protection 155:1-8, 105927 (https://doi.org/10.1016/j.cropro.2022.105927).
- Potter, D.A. 1998. Destructive Turfgrass Insects-Biology, Diagnosis, and Control. Ann Arbor Press, Chelsea, Mich.
Albrecht M. Koppenhöfer (a.koppenhofer@rutgers.edu) is an Extension specialist in the Department of Entomology, Rutgers University, New Brunswick, N.J. Olga S. Kostromytska is an Extension assistant professor in the Stockbridge School of Agriculture, University of Massachusetts, Amherst, Mass. Lemma Ebssa is a senior data scientist at ICF Inc., Fairfax, Va