Several species of lance nematodes are known to feed on warm-season turfgrasses. The most common is Hoplolaimus galeatus. Photos by William Crow
Editor’s note: This research was partially funded by GCSAA through a grant from the GCSAA Foundation.
The three most important kinds of nematodes on warm-season turfgrasses on golf courses — the “big three” — are sting, root-knot and lance nematodes. Lance nematodes are not the most directly damaging of these three nematodes, but they are the most difficult to manage and also the most difficult to diagnose. Concern about lance nematode has been increasing among golf course superintendents, particularly as some of our newer nematicides can provide good results on the other two major kinds of nematodes, but not lance.
Several species of lance nematodes are known to feed on warm-season turfgrasses. However, the most common, and the species that I conduct research on, is Hoplolaimus galeatus. Be aware that when I am writing about lance nematodes, I am specifically referencing H. galeatus, but it is possible that other species of lance nematodes on turf might behave differently.
On turf, lance nematodes are what nematologists call “migratory endoparasites.” This means they tunnel into turf roots, tunnel around within the roots feeding as they move from cell to cell, and can tunnel back out again. Activity by these nematodes destroys root cells, disrupts root tissue and creates entry points for root pathogens.
Lance nematodes are found in the soil and within turf roots, so systemic nematicides that kill nematodes within roots would be ideal against these nematodes. This is a reason that fenamiphos (Nemacur) was so effective against a wide array of nematodes — it had both systemic and contact activity. Unfortunately, in 2021, a decade after the withdrawal of Nemacur, we do not have any true systemic turfgrass nematicides available.
The three most commonly used nematicides for nematodes on warm-season golf grasses are fluopyram (Indemnify, Bayer), abamectin (Divanem, Syngenta; Todal, Quali-Pro; Nemamectin, RightLine), and 1,3-dichloropropene (Curfew), where these can be used. Note that 1,3-dichloropropene will kill lance nematodes outside roots at the time of application, but it does not kill those inside roots and has no residual activity, so it is of limited use against lance nematodes. Abamectin kills lance nematodes it contacts, but only those outside the roots and, because of its limited movement, only in the extreme upper portion of the soil profile. While fluopyram is effective against most kinds of nematodes, lance nematodes are one of the few kinds on which this nematicide does not work. Therefore, none of our current nematicide options works well against lance nematodes.

These roots have been damaged by lance nematodes, whose activity destroys root cells, disrupts root tissue and creates entry points for root pathogens.
In our turfgrass nematicide trials with current nematicides, we typically observe increases in numbers of lance nematodes over time from repeated applications. At the University of Florida Nematode Assay Lab, we have observed sharp increases in the numbers of lance nematodes recovered from bermudagrass greens since the launch of fluopyram and abamectin nematicides in 2016.
Despite poor efficacy of current nematicides against lance nematodes, it is not uncommon to observe positive turf response from nematicide applications. We believe this is due to control of other plant-parasitic nematodes present and possibly impacts on fungal pathogens and turf pests. Sometimes we recover high numbers of lance nematodes from healthy-appearing turf.
Diagnosing lance nematodes is also difficult because of their migratory, endoparasitic lifestyle. Are the numbers recovered from soil an accurate assessment of the population? When should we sample for lance nematodes? What should the “thresholds” be? I have long thought that the thresholds we were using needed revision. The UF Nematode Assay Lab started using a mist extraction method for extracting root-knot nematodes from turf plugs and roots. On warm-season grasses, mist extraction is superior to soil extraction for root-knot nematodes. Lance nematodes are also recovered by mist extraction. Will mist extraction results be superior to those of soil extraction for lance nematodes, too?
Our two-year experiment, funded by the GCSAA Chapter Cooperative Research Program with the Florida GCSA, sought insight into the following questions:
- What action thresholds should we be using for lance nematodes on warm-season turf?
- Which extraction method is most accurate for lance nematodes?
- When is the best time to sample for lance nematodes?
Experiment details
To answer these questions, we selected two test sites. One was TifEagle bermudagrass at the University of Florida Research and Education Center in Fort Lauderdale (South Florida), and the other was Sunday bermudagrass at the University of Florida Plant Science Unit in Citra (North Florida). Both sites were naturally infested with Hoplolaimus galeatus, having counts exceeding existing thresholds at the beginning of the trial, although numbers were generally higher at the North Florida site.
At each site, we laid out 120 4.5-square-foot (0.4-square-meter) plots. At each site, 60 plots were untreated, and 60 plots were treated with Indemnify (17.1 fluid ounces per acre; 1.25 liters per hectare) each March and October, and Divanem (12.2 fluid ounces/acre; 0.9 liters/hectare) each May, June, July and August. The nematicides were not applied in an attempt to control lance nematode. Our previous research indicated that neither Indemnify nor Divanem was likely to be effective against lance nematodes. Rather, we included these nematicides to see how lance nematodes respond when we effectively control other plant-parasitic nematodes and not lance nematodes.
Nematode and root samples were collected four times per year: March, June, September and October. Nematode samples were four 1.5-inch-diameter (3.8-centimeter-diameter) and 2.5-inch-deep (6.4-centimeter-deep) plugs from each plot. Nematodes were extracted from each sample using two extraction methods: the typical soil extraction using centrifugal flotation (2), and mist extraction from turf plugs and roots (1). The soil was knocked off the plugs, and nematodes were extracted from a 100-cubic-centimeter soil subsample per plot. The four plugs with adhering roots and thatch were washed and placed in a mist chamber for 72 hours to collect lance nematodes and root-knot nematodes exiting roots. Nematodes collected from each sampling method were counted separately. Two root samples were 1.5 inches (3.8 centimeters) in diameter and 6 inches (15 centimeters) deep from each plot. Roots from the two cores per plot were combined, extracted manually in water with a sieving technique, and measured using WinRHIZO equipment and software.
Turf measurements were collected every two weeks, except when prevented by topdressing, hurricane, winter freeze, flood and pandemic-related events. Measurements taken were turf quality (1-9), normalized difference vegetation index (NDVI; turf stress) and TruFirm (hardness). Data from multiple measurements per month were averaged, and the average monthly values were used for analysis. In general, turf quality and NDVI yielded similar results, and because turf quality is more readily relatable, it will be used in the discussion. TruFirm measurements did not yield useful information and will not be discussed.
General observations
Lance nematode counts were generally higher at the North Florida site (Figure 1, below) than at the South Florida site (Figure 2, below). While lance nematode counts from soil in South Florida started off in excess of the “high risk” thresholds used at the time by the UF Nematode Assay Lab, their numbers decreased throughout the trial. However, in North Florida, the lance nematode numbers started off high and remained high throughout. Another observation from the North Florida site was that, as in previous trials, numbers of lance nematodes recovered from soil got much higher in the nematicide-treated plots than in the untreated plots. At the North Florida site, there were fairly low numbers of root-knot nematode, ring nematode and a few other kinds of plant-parasitic nematodes present. However, at the South Florida site, there were abundant (in excess of “high risk” thresholds) root-knot nematodes present.
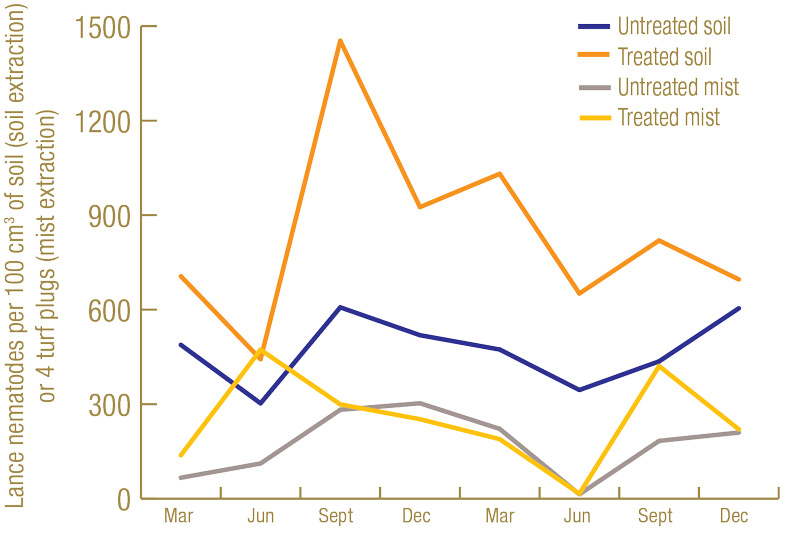
Figure 1. Average number of lance nematodes recovered by centrifugal flotation from 100 cubic centimeters of soil, or by mist extraction from four 1.5-inch-diameter (3.8-centimeter-diameter) turf plugs over the course of the experiment at the North Florida site from 60 untreated or 60 nematicide-treated plots.

Figure 2. Average number of lance nematodes recovered by centrifugal flotation from 100 cubic centimeters of soil, or by mist extraction from four 1.5-inch-diameter (3.8-centimeter-diameter) turf plugs over the course of the experiment at the South Florida site from 60 untreated or 60 nematicide-treated plots.
In the nematicide-treated plots, after reaching a high point of around 1,450 lance nematodes per cubic centimeter of soil in September 2019, lance nematode numbers trended downward for the remainder of the trial. This population reduction was likely due to a fungal parasite we observed infecting large numbers of lance nematodes in the nematicide-treated plots. By the end of the trial, an estimated three-quarters of the lance nematodes in some of the nematicide-treated plots were dead, and their bodies were completely filled by this fungus. When counting the lance nematodes, only live, noninfected lance nematodes were included in the analysis. Therefore, the total lance nematode counts likely remained fairly constant after September 2019, but the numbers of live ones capable of damaging turf steadily decreased. Currently, the identity of this lance-nematode-killing fungus is unknown, but we plan to conduct research on it in the future. We did not observe fungal infection at the South Florida site, so this fungus is likely not the cause of decreases in lance nematode abundance at that location.
Root lengths were greater on the Sunday bermudagrass in North Florida than on the TifEagle bermudagrass in South Florida (Figure 3, below). Having more roots to feed on may have contributed to higher lance nematode counts in North Florida than South Florida. Turf quality was greater on the TifEagle bermudagrass in South Florida than on the Sunday bermudagrass in North Florida (Figure 4, below), possibly because lance nematodes were fewer at the South Florida site than at the North Florida site.
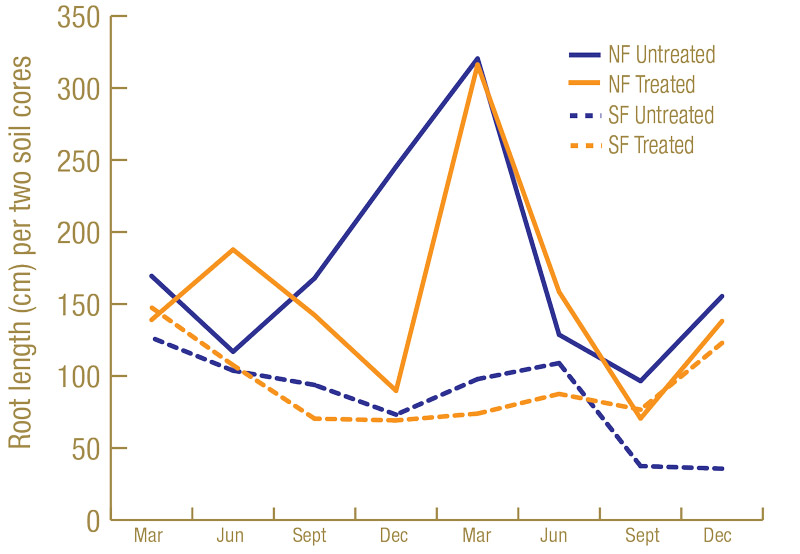
Figure 3. Average root length (centimeters) from two cores per plot from 60 untreated plots or 60 nematicide-treated plots in North Florida and South Florida trial sites.
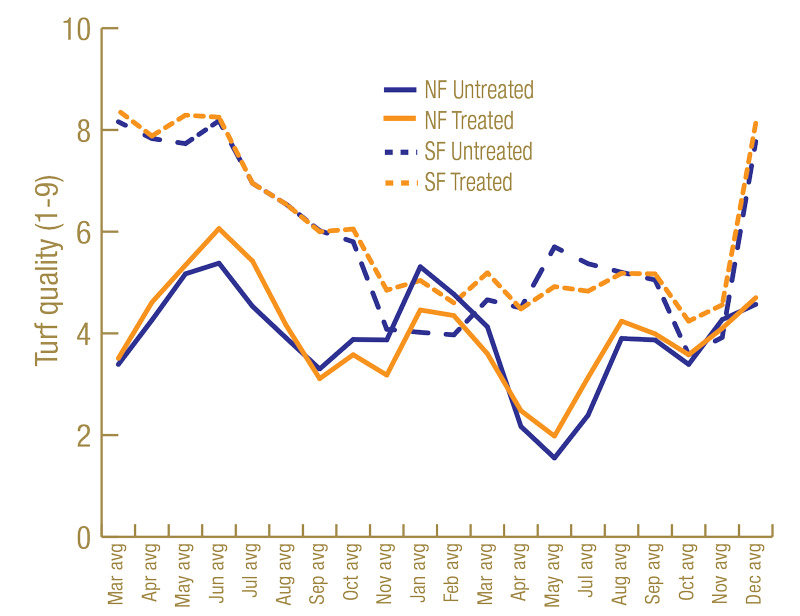
Figure 4. Average turf quality (1-9) from 60 untreated plots or 60 nematicide-treated plots in North Florida and South Florida trial sites.
Analysis
To answer the research questions, we used a combination of regression analysis and observation. Monthly average turf quality for each month were regressed on the nematode counts using both extraction methods, for the current and all preceding sampling dates. Similarly, turf root length at each sampling date was regressed on the lance nematode counts for the current and all preceding sampling dates.
Sampling and extraction
Regression analysis was used to determine which extraction method was better at estimating lance nematode impacts on turf quality and root lengths. Regression analysis is used to determine how related two factors are. For example: Figure 5 (below) is the regression of turf quality at the North Florida site in July 2020 on the number of lance nematodes recovered from soil extraction in March 2020.
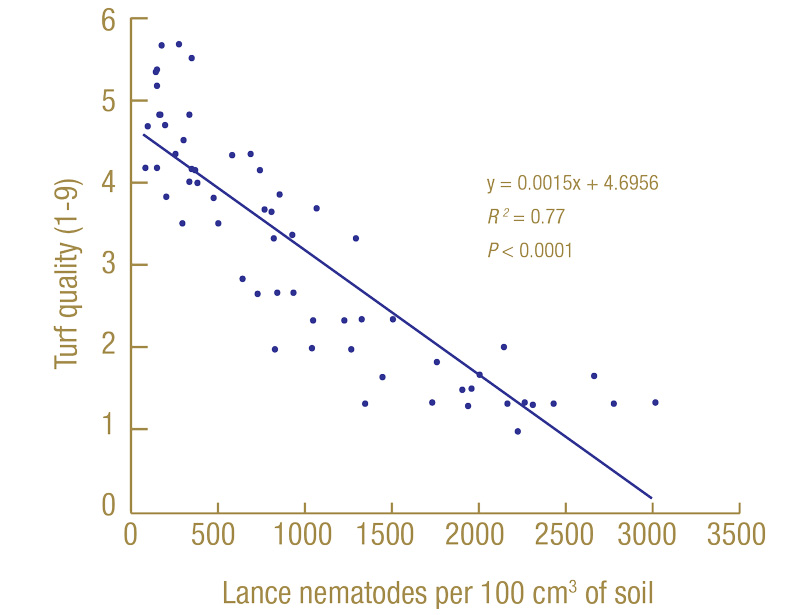
Figure 5. Linear regression of turf quality (1-9) in July 2020 on the number of lance nematodes recovered by soil extraction in March 2020 from 60 nematicide-treated plots at the North Florida site.
The line slopes downward, indicating that as nematode numbers (the x-axis) increased, the turf quality (y-axis) decreased. The slope of the line (y = 0.0015x + 4.7) can be used to determine the expected turf quality if no lance nematodes were present (4.7) and how many nematodes it would take for turf quality to be reduced by 1 full point (667 lance nematodes per 100 cubic centimeters of soil). The R2 value tells us how much of the variability in turf quality among plots can be explained by lance nematodes (in this case 0.77, or 77%); the larger the number, the better, so 1.00 is as good as it gets. The P tells us how statistically significant the association is. The smaller the P, the better; P < 0.0001 is as good as it gets, and anything greater than 0.1 we considered not significant. We ran hundreds of individual regressions, so I will not show them all here, but I will give you a summary of what we found.
The number of lance nematodes recovered by soil extraction was better for estimating turf quality than the number of lance nematodes recovered using mist extraction. That is not to say that the number of lance nematodes recovered by mist extraction was never useful for predicting turf quality, but significant interactions (good Ps) were not as common, and the associations (the R2s) were usually not as strong as from soil extraction.
Editor’s note: Author William T. Crow, Ph.D., imparts even more nematode knowledge in Managing root-knot nematodes in warm-season turfgrass, outlining the life cycle and behavior of root-knot nematodes, a new method for diagnosis, and current tools for control.
The nematode counts from nematicide-treated plots were better at predicting turf quality than were the counts from untreated plots. We attribute this to two factors: 1) Lance nematode counts in some of the treated plots got much higher than in the untreated plots and gave us a larger range on the x-axis. Larger x-axis ranges generally make for better regressions. 2) The nematicides provided control of other nematodes, reducing their confounding influence on turf quality and root length.
We also found that the R2s (how good the nematode counts were at predicting turf quality) generally increased for three to six months after sampling. This means that a lance nematode sample is more effective at predicting turf quality several months in the future than at estimating current damage. It is best to collect the nematode sample three to six months before you expect nematode damage to occur. From the results of this study, the best times to sample for lance nematodes in Florida (and likely anywhere from Dallas to Atlanta and south) are September and March.
Tolerance limit and thresholds
A few nematodes are typically unlikely to cause damage. However, as nematode numbers increase, they get to a number where they start having a measurable negative impact. Nematologists refer to this number as the “tolerance limit.” The actual tolerance limit is unknown; it varies from green to green, from green edge to green center, from month to month, etc. However, with enough data, we can estimate that it is likely to be within a given range. This range is what I use for my “moderate risk threshold.” Below this range I designate as “low risk,” and above it as “high risk.”
In this study, we observed that increasing turf quality was often associated with increasing numbers of lance nematodes, until the lance nematode counts reached the tolerance limit. This doesn’t mean that the nematodes made the grass better; rather, it means that healthier grass provides more food for the nematodes, allowing their numbers to increase. The grass was affecting the nematodes more than the nematodes were affecting the grass until the tolerance limit was reached. However, after reaching the tolerance limit, turf quality decreased with increasing numbers of lance nematodes. This tolerance limit varied, but we were looking for the range that the individual tolerance limits generally fell into and not a specific value.
The tolerance limit is much more difficult to determine on plots where nematicides were not used; the influence of other kinds of nematodes in the untreated plots made the data much more variable (low R2s). Therefore, only the nematicide-treated plots were used for determining thresholds.
For illustration, we will look at a specific example. Figure 6 (below) shows the average turf quality of nematicide-treated plots in North Florida in July 2020 associated with the average number of lance nematodes recovered from soil and grouped in increments of 100 from March 2020.
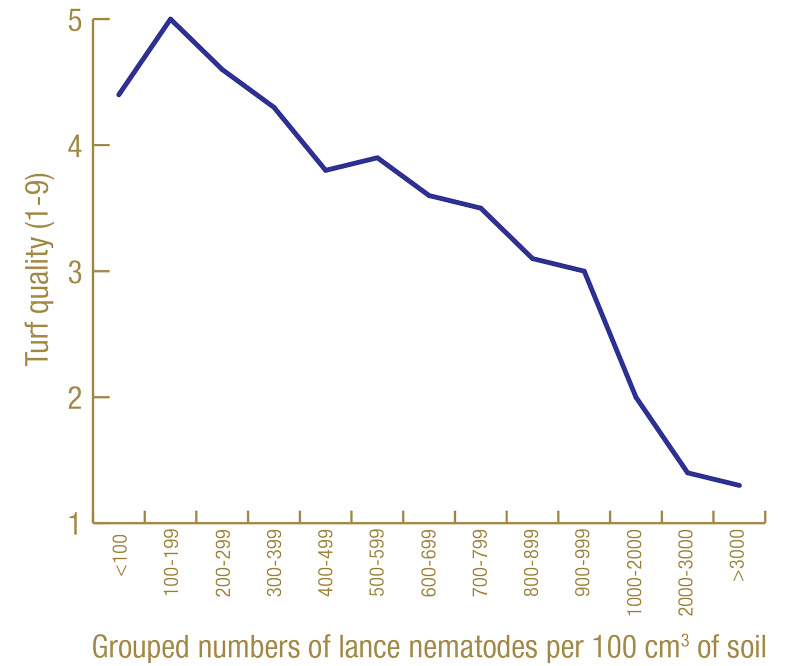
Figure 6. Average turf quality (1-9) in July 2020 related to grouped numbers of lance nematodes recovered by soil extraction in March 2020 from 60 nematicide-treated plots at the North Florida site.
Turf quality increased with increasing numbers of lance nematodes until somewhere around 100 to 200 nematodes per 100 cubic centimeters of soil. However, after that, turf quality steadily decreased with increasing nematode numbers. In this example, the tolerance limit appears to be around 200 lance nematodes per 100 cubic centimeters of soil. Again, I am not going to show all of these graphs, but in most cases, the tolerance limit appears to be around 100 to 300 lance nematodes per cubic centimeter of soil, and that is what I have adopted as my moderate risk threshold.
Unfortunately, current nematicide options are generally ineffective for managing lance nematodes on golf course bermudagrass. Overreliance on these nematicides can lead to increasing problems with lance nematode. However, I want to encourage the reader not to get too depressed. I have been working with some promising new nematicides for the management of lance nematodes and expect some of these to become available in the next couple of years.
Funding
This work was supported by GCSAA through a grant from the GCSAA Foundation and the Florida Golf Course Superintendents Association.
Acknowledgments
We would like to thank the GCSAA Foundation and the Florida GCSA for funding this research. We also want to thank the UF Landscape Nematology team (Chris Green, Maria Mendes, Tom Bean, Mengyi Gu, Benjamin Waldo and Matt Walkup) for all of their hard work sampling, extracting nematodes, counting nematodes, processing roots, etc. Finally, we want to acknowledge the staff at the UF Plant Science Unit (Mark Kann and his team) and the UF Fort Lauderdale Research and Extension Center (Karen Williams and her team) for taking care of the turf plots and providing help when we needed it.
The research says ...
- Both soil and mist extraction can be used to diagnose lance nematodes from bermudagrass, but soil extraction is generally better.
- The old lance nematode thresholds needed revision. The new thresholds for lance nematodes on bermudagrass (and other warm-season turfgrasses) adopted in 2021 by the UF Nematode Assay Lab are: <100 per 100 cubic centimeters of soil = low risk; 100-299 per 100 centimeters of soil = moderate risk; and >299 per 100 cubic centimeters of soil = high risk.
- It is best to sample for lance nematodes three to six months before you expect damage to occur. In most of the South, this is September and March.
Literature cited
- Crow, W.T., A. Habteweld and T. Bean. 2020. Mist chamber extraction for improved diagnosis of Meloidogyne spp. from golf course bermudagrass. Journal of Nematology 52:e202096 (https://www.exeley.com/journal_of_nematology/doi/10.21307/jofnem-2020-096).
- Jenkins, W. 1964. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter 48:692.
William T. Crow is a professor of nematology at the University of Florida.