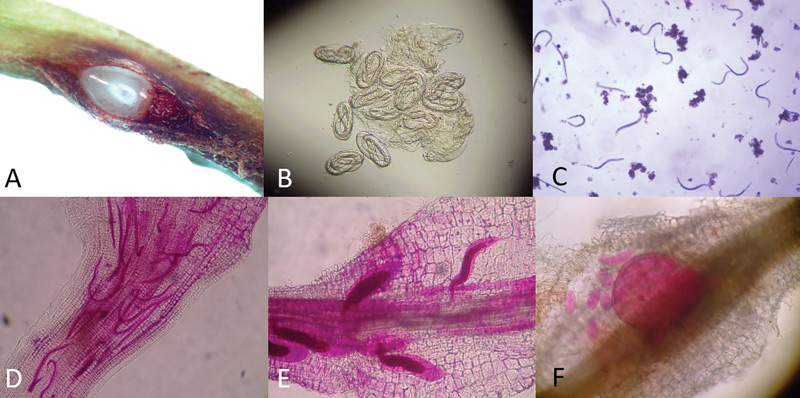
Figure 1. The life cycle of a root-knot nematode. A: Split in a grass root revealing a female root-knot nematode and her eggs (stained red) within. B: Root-knot nematode eggs with second-stage juveniles (J2) inside them ready to hatch. C: Root-knot nematode J2 that are mobile in soil. D: Root-knot nematode J2 (stained red) that have entered a bermudagrass root. E: Root-knot nematode J3 (stained red) inside a bermudagrass root. F: Adult root-knot nematode (stained red) laying eggs inside a bermudagrass root. Photos by William Crow
Root-knot nematodes cause extensive damage on warm-season turfgrasses, but until recently, their damage was largely misdiagnosed. I was drinking coffee with USGA agronomist Steve Kammerer recently and discussing the impacts of root-knot nematodes on turf roots when Kammerer made the comment that dealing with root-knot nematodes is like “chasing ghosts.” Like Peter Venkman (Bill Murray’s character) and his colleagues in “Ghostbusters,” the University of Florida Landscape Nematology and Nematode Assay labs have been chasing these tiny, subterranean ghosts and have learned a lot about them, particularly over the past 10 years. We have even managed to catch a few.
To begin, I want to state that there are around 100 species of root-knot nematodes described and several that are known to infect turfgrasses in the United States. Of those that infect turf, some infect only cool-season grasses, some infect only warm-season grasses, and some infect both. Because of where I am located, Florida, I only study the species that infect warm-season grasses. The two root-knot nematode species that most commonly infect warm-season turfgrasses on golf courses in the U.S. are the grass root-knot nematode (Meloidogyne graminis) (1) and the Maryland root-knot nematode (Meloidogyne marylandi). Unless otherwise noted, when I refer to root-knot nematodes from this point forward, I will be referring to these two species.
Since I started working with turfgrasses 20-plus years ago, I have often seen bermudagrass that was declining, yellowing, blotchy and slow to recover from damage. Sometimes we would sample and find high numbers of root-knot nematodes in the soil, and other times few or none. Sometimes we would find few or none in the soil, but find that the roots were full of them. Often, we would get a big positive response from a nematicide despite not finding many nematodes in soil. I became convinced that our existing ghost-chasing methods were not working well for root-knot nematodes.
The nematode life cycle
Any good ghostbuster will tell you that to effectively catch a ghost, you need to study its behavior and know its haunts. Root-knot nematodes begin as eggs that, depending on the grass host, will be mostly inside roots (bermudagrass, seashore paspalum) or on the root surface (zoysia, bahiagrass). The second-stage juvenile (J2) is the life stage that hatches from the egg. The J2 are small, worm-shaped and mobile. The J2 exit the root they were born in (or on) and then find a new root to infect. Typically, they enter a growing root tip.
Within the new root, the J2 migrate to cortical cells and start injecting hormones that cause a feeding site to develop. After initiating the feeding site, the nematode no longer moves. It molts into a J3 and then a J4, getting larger and more swollen with each molt. After its final molt into an adult, the female root-knot nematode lays up to several hundred eggs in an egg mass inside or on the root.
The time between when a root-knot nematode egg hatches and when it becomes an egg-laying adult is about three weeks, but that period can be longer or shorter depending on environmental conditions. In Florida, conditions are favorable for root-knot nematode activity year-round, although it will slow in winter. Farther north, root-knot nematodes may be completely inactive during the colder months.
The most important part of this life cycle (Figure 1, above) is that the only time in its life that the root-knot nematode is in the soil is when it is a J2 after it has hatched and left a root and before it goes inside another one. Usually this is only a few hours to a couple of days. So the number of J2 recovered from soil may only represent the tip of the iceberg compared with the number within the roots. Perhaps a way to estimate root-knot nematodes within roots would be more accurate than J2 counts from soil.
Another relevant thing we discovered is that, on warm-season turf, root-knot nematodes tend to proliferate in the upper inch of the soil profile and thatch. Relatively fewer root-knot nematodes are found deeper in the soil profile. This is different from most other turfgrass nematodes that predominate 2 to 4 inches deep. In a normal soil assay — nematologists call this the Jenkins method (3) — we throw away the turf, thatch, root material and upper soil, and it turns out we throw away most of the root-knot nematodes, too. A method to include the turf plug in the sample should give a more accurate assessment.
Designing a nematode ‘ghost trap’
Now that we know more about where to find our ghost and how it behaves, we need a root-knot nematode version of the “muon ghost trap” to catch them. After trying a few different methods, we ended up using a modified mist chamber method (2).

Figure 2. A (top): The University of Florida Nematode Assay Lab mist chamber. B (middle): The middle shelf holds the funnels and samples. A flask is placed below each funnel to collect the nematodes. C (bottom): Samples are four turf plugs with adhering roots.
The UF Nematode Assay Lab mist chamber (Figure 2) is a plexiglass box with mist rails attached to the top and a middle shelf to hold samples. Holes are cut into the middle shelf to hold funnels, and screen rings are used to hold the sample. The sample is placed onto a coffee filter, and the filter is placed onto the ring. Flasks are placed below each funnel to catch the nematodes. The mist comes on for about 10 seconds each hour. As the root-knot nematode eggs hatch, the J2 exit the roots and get washed onto the coffee filter. The nematodes then crawl through the filter and are washed into the flask below. Samples are incubated in the mist chamber for 72 hours.
Samples for nematode mist extraction are different from those for normal soil extraction. To learn how to collect this kind of sample, I recommend watching our how-to video:
The samples are four turf plugs that are 1.5 inch (3.8 centimeters) in diameter and 2.5 inches (6.4 centimeters) deep. The soil is washed from the plugs, and the plugs with adhering thatch and roots are placed in the mist chamber.
Results and discussion
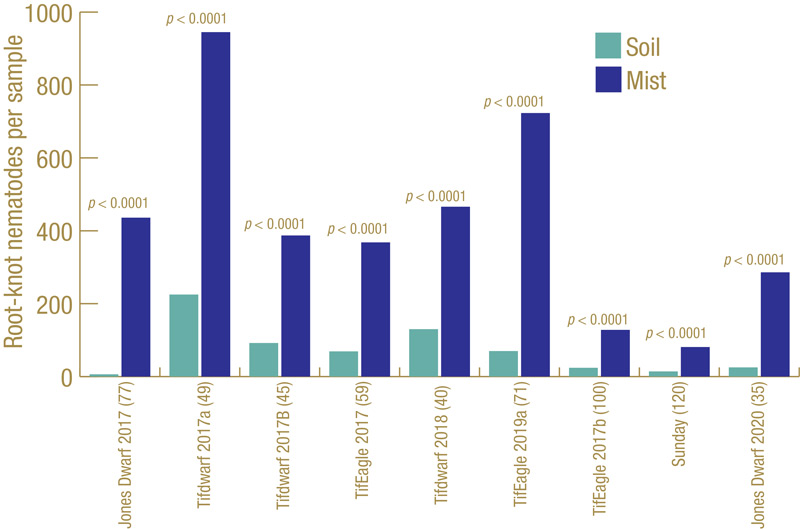
Figure 3. Root-knot nematodes recovered from small-plot trials on different bermudagrass cultivars at different locations in multiple years, comparing standard soil extraction with mist extraction.
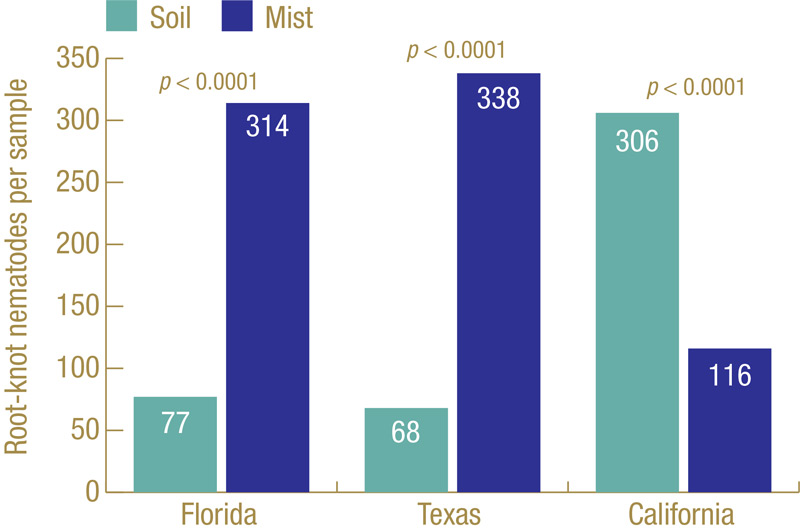
Figure 4. Root-knot nematodes recovered from golf course diagnostic samples submitted to the UF Nematode Assay Lab over a two-year period that were extracted by standard soil extraction or mist extraction. Samples are from bermudagrass (Florida and Texas) or bentgrass/Poa (California).
How well does our ghost trap work? Figure 3 (above) shows the results from multiple field trials at different locations on different cultivars of bermudagrass over multiple years. Each trial had many small plots, with 45 to 120 plots per trial, and root-knot nematodes J2 were extracted from soil and also from roots using the mist chamber. In every trial, mist extraction yielded significantly greater numbers of root-knot nematode J2. Figure 4 shows results from diagnostic golf turf samples submitted to the UF Nematode Assay Lab from three states (Florida, Texas, California) over a two-year period in which both extraction methods were used. The Florida and Texas samples all came from bermudagrass; the California samples all came from cool-season grasses. Mist extraction from bermudagrass in Florida and Texas had greater recovery of root-knot J2 than soil extraction did. However, the opposite was observed from the cool-season California samples.
Why were the California samples different? The most common root-knot nematode species on cool-season turf in California are Meloidogyne naasi and Meloidogyne minor (4), and perhaps these species behave differently than those more common. It could also be that the nematodes behave differently on the different types of grass. Whatever the reason, while mist extraction is generally better for diagnosing root-knot nematodes on bermudagrass and other warm-season grasses, that may not be the case for cool-season grasses.
Another point of consideration is that while mist extraction is generally better than soil extraction on warm-season grasses, and while soil extraction was generally better than mist extraction from the California samples, there are exceptions. In some cases, soil extraction yields higher root-knot nematode counts than mist on warm-season turf, and vice versa on cool-season turf. This may be due to conditions that trigger most of the eggs to hatch at once or other unknown factors.
While one method may work better than the other most of the time, it is a good idea to do both if you want to be certain of the assay results.
Since implementing mist extraction for our assessments of root-knot nematodes on warm-season grasses, we have been correlating the nematode counts with turf and root damage and have developed revised risk thresholds using these numbers. The new risk thresholds for root-knot nematodes used by the UF Nematode Assay Lab implemented at the beginning of 2021 are shown in Table 1, Revised risk thresholds for root-knot nematodes. These may be further updated over time with new research and field observations. For soil counts less than 100 per 100 cubic centimeters (6.1 cubic inches) of soil, we do not give a diagnosis because of the inaccuracy of the method, and we ask that you send in separate samples for mist extraction. For soil counts of 100 or more, we assume that with that many in the soil, the roots are likely full of nematodes too, and we are more confident in providing a diagnosis.
Mist extraction is a supplement to, but not replacement for, soil extraction. While this new method is most accurate for diagnosing root-knot nematodes on warm-season turf, it is not a good extraction method for most of the other kinds of turfgrass nematodes (sting nematode, etc.). Therefore, it is important that you still send in standard samples for soil extraction.

Figure 5. A nematicide experiment targeting sting nematode where no impacts on sting nematode were observed but the turf improved anyway. It was later discovered that the turf response was from control of root-knot nematodes that did not show up in the nematode soil assays.

Figure 6. An ultradwarf bermudagrass green with yellow blotching caused by root-knot nematodes.
Now that we have an improved ghost trap, some of the former ghostly mysteries are explained. A reason we would sometimes get positive turf responses from nematicides when we could find no effects on nematodes in the soil was because we were impacting root-knot nematodes we didn’t know were there (Figure 5, above). Yellow blotches on ultradwarf bermudagrass greens from unknown causes, we now know, can be caused by root-knot nematodes (Figure 6, above). Unexplained poor root systems on greens and slow recovery from damage are often caused by root-knot nematodes.
We have found that root-knot nematodes are easily and commonly spread in sprigs. Fumigation during construction kills off many of the root-knot nematodes’ natural enemies. So, when new grass is sprigged into fumigated soil, root-knot nematodes can be sown at the same time and allowed to reproduce unhindered until the soil ecology is restored. One of the common causes of slow establishment of warm-season turf is root-knot nematodes (Figure 7, below).
Nematode control methods
OK, ghostbusters, now that we better understand how root-knot nematodes behave, what they do, and how to catch them ... we need a proton pack to zap them with. Because root-knot nematodes spend most of their life inside roots, it would be nice if we could use a systemic nematicide like Nemacur (which the EPA banned in 2011) that would kill nematodes inside roots. Unfortunately, none of the turf nematicides currently available are systemic, and we have to use contact nematicides targeting the J2 after it leaves a root and before it enters another one. We know that on warm-season turf, root-knot nematodes are mostly in the top inch of the soil profile, so we need a nematicide that is not too mobile or it will wash past them. Because contact nematicides do not kill root-knot nematodes inside roots, the nematicide needs to be present at an effective concentration long enough to target multiple generations of J2 as they exit the roots.

Figure 7. A recently fumigated and sprigged ultradwarf bermudagrass green struggling to establish because of infestation with root-knot nematodes.
University of Florida research indicates that abamectin (Divanem, Syngenta; Todal, Quali-Pro; Nemamectin, RightLine) and fluopyram (Indemnify, Bayer) are effective tools for managing root-knot nematodes on warm-season turf. Abamectin is nearly immobile in soil, so it stays in the top inch of soil where root-knot nematodes predominate. The half-life of abamectin in soil is two to four weeks, so to maintain an effective concentration long enough to impact multiple generations of root-knot nematodes, multiple applications at two- to four-week intervals are required. Fluopyram is moderately mobile, so after a time it will move downward and past the root-knot nematode target zone. In our trials, we typically get four to eight weeks of root-knot nematode benefit from an Indemnify application.
For root-knot nematode control, both nematicides can be used in rotation or individually. UF research results indicate that an application of the full rate of Indemnify followed by four applications of the full rate of an abamectin nematicide at four-week intervals provides maximum control of root-knot nematodes while adhering to the product labels.
If cost or other factors prevent using both nematicides, the decision on which to use can be assisted by the presence or absence of other kinds of nematodes. Given that abamectin has limited practical effect on other turf nematodes because of its poor movement, it may be the best choice if root-knot nematodes are the only nematode of concern. In this case, four applications of the full rate of the abamectin product you are using would likely yield good results. However, if other plant-parasitic nematodes, like sting nematode, are also present, Indemnify might be a better choice because it can help control nematodes deeper in the soil along with the root-knot nematodes. In this case, two applications of the half-rate of Indemnify, six weeks apart, should provide good results.
Thank you for bearing with me through some 1980s nostalgia (apologies to you youngsters). I ain’t afraid of no ghost!
The research says ...
- Consider the life cycle of root-knot nematodes when developing a control strategy.
- Mist extraction from turf plugs is generally superior to soil extraction for diagnosis of root-knot nematodes on warm-season grasses in the Southeast, but mist extraction is a supplement to, not a replacement for, soil extraction.
- On warm-season turf, root-knot nematodes are mostly in the top inch of the soil profile, so we need a nematicide that is not too mobile or it will wash past them. The nematicide needs to be present at an effective concentration long enough to target multiple generations of second-stage juveniles as they exit roots.
- Root-knot nematodes can be effectively managed with rotations of fluopyram and abamectin nematicides.
Literature cited
- Crow, W.T., A. Habteweld and T. Bean. 2020. Mist chamber extraction for improved diagnosis of Meloidogyne spp. from golf course bermudagrass. Journal of Nematology 52:e202096 (https://www.exeley.com/journal_of_nematology/doi/10.21307/jofnem-2020-096).
- Crow, W.T. 2019. Grass root-knot nematode: Meloidogyne graminis. University of Florida, Gainesville, Fla. (https://entnemdept.ufl.edu/creatures/NEMATODE/grass_rootknot.html).
- Jenkins, W. 1964. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter 48:692.
- McClure, M.A., C. Nischwitz, A.M. Skantar, M.E. Schmitt and S.A. Subbotin. 2012. Root-knot nematodes in golf greens of the western United States. Plant Disease 96:635-647.
William T. Crow is a professor of nematology at the University of Florida.