
Take-all root rot patch symptoms on an ultradwarf bermudagrass putting green. Photos by Bruce Martin
Take-all root rot is a common but potentially devastating disease on bermudagrass putting greens that has been diagnosed frequently in the past several years in the Carolinas and elsewhere in the southern United States. One name for the disease is take-all root rot, but it is also known as bermudagrass decline (when in bermudagrass) and root decline of warm-season grasses.
The term root decline describes roughly a continuum of non-distinct visual symptoms including thinned turf stands, irregular chlorotic patches and distinct circular patches of diseased turf. Symptoms begin as irregular, off-color patches that initially become yellow and later lead to death and necrosis of lower leaves. Symptoms on leaves are typical of those induced by nematodes or other root pathogens, and leaf spots and other infestations are secondary in nature. Root systems of infected bermudagrass are shallow and discolored with distinctive dark-colored lesions on the roots, leading to eventual brown-to-black rotting of the entire root and sometimes the stolons and rhizomes. Microscopically, the causal fungi produce dark brown-to-black runner hyphae on rhizomes, roots and stolons of infected plants.
These symptoms have been associated with the pathogen Gaeumannomyces graminis var. graminis (GGG), but research has shown that similar but distinct fungi are associated with root diseases in warm-season grasses. So, the term root decline may be most appropriate when referring to these diseases in a broad sense, whether they occur in bermudagrass, St. Augustinegrass, centipedegrass, kikuyugrass, zoysia, seashore paspalum or other hosts. When the causal agent is purported to be GGG, we refer to the disease as take-all root rot.
To be clear, take-all root rot refers to the root disease occurring in warm-season grasses caused by GGG, while take-all patch refers to a similar disease primarily occurring in cool-season grasses, including Agrostis (bentgrasses), and less frequently in fescues and bluegrasses. The causal agent of take-all patch is G. graminis var. avenae (GGA). Take-all patch (previously known as Ophiobolus patch) was the first known and is the most studied of the root-rot diseases in grasses, occurring in temperate climates around the world and most commonly in cool-humid regions in the United States. Two other varieties of G. graminis cause root-rot disease in crop plants: G. graminis var. tritici (GGT), which causes take-all in wheat, barley and rye, and G. graminis var. maydis, which causes take-all in maize, sorghum and other cereals.
 |
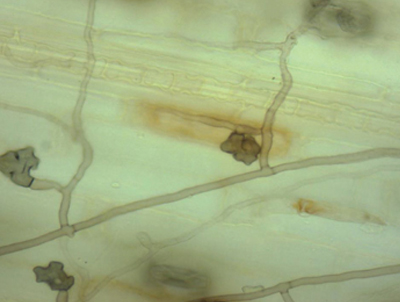 |
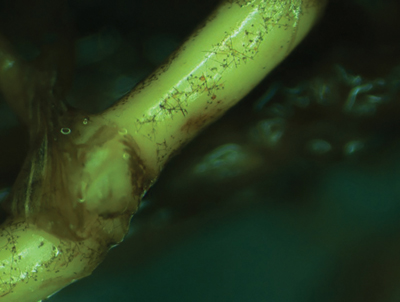
Left: A bermudagrass stolon showing ERI fungi typical of Gaeumannomyces graminis var. graminis, with lobate hyphopodia just visible. Right: Close-up microscopic view of lobate hyphopodia typical of Gaeumannomyces graminis var. graminis.
Genetic differences
Modern molecular methods are allowing scientists to study these organisms in much greater detail than was possible just a few years ago. Recent work has shown that the varieties of Gaeumannomyces graminis (var. graminis, avenae, tritici and maydis) are bona fide biological species based on their genetic and morphological differences. So, you will likely see plant pathologists begin to refer to the causal agents as G. graminis, G. tritici, G. avenae and G. maydis, doing away with the subspecies variety and elevating it to the species level. This result should help improve our understanding of these organisms and their associated host plants, with less confusion over the use of varieties of G. graminis.
In addition, an excellent recent study of take-all pathogens from Poaceae (the grass family) has shown that GGG is highly diverse genetically and is likely a species complex, and actually includes 14 cryptic species, some of which (including GGG or G. graminis) occur on turfgrasses. So, the chore for turf pathologists now is to bore down in more detail with fungal isolates tentatively identified as GGG to determine where they may fit among the newly described Gaeumannomyces species.
Causal agents
To complicate matters further, as we study root diseases more, we are likely to find similar but distinct causal agents that induce nonspecific root rots in both cool- and warm-season turfgrasses and other hosts. For example, research at Mississippi State by Philip Vines, M.S., and Maria Tomaso-Peterson, Ph.D., has identified new species of Magnaporthiopsis and other fungi associated with “summer root decline” in bermudagrass.
The fungi causing take-all root rot are in the class Ascomycetes, in the fungal family Magnaporthaceae. As with necrotic ring spot and summer patch, infected roots exhibit the classic ERI sign of darkly pigmented fungal hyphae. ERI stands for “ectotrophic root-infecting,” and refers to the habit of these fungi to colonize the root epidermis, cortex and ultimately the stele with dark hyphae that grow linearly along the root and are easily observed microscopically.
These observed ERI signs are not diagnostic of a particular disease, and further examination is required for better knowledge of a purported causal agent. One way to help distinguish causal agents is to look at the shape of specialized cells on the ends of certain hyphae: The hyphal ends may be a simple swelling of the hypha, or the ends may be more or less differentiated into finger-like outlines of the specialized cells. These structures are called hyphopodia. With Magnaporthiopsis, Ophiosphaerella, GGA and GGT, the ends of the hyphae are only slightly swollen and are called simple hyphopodia. With GGG, the hyphopodia are ornately lobed in general and are termed lobed hyphopodia. These structures are visible with a good hand lens, but are better observed microscopically.
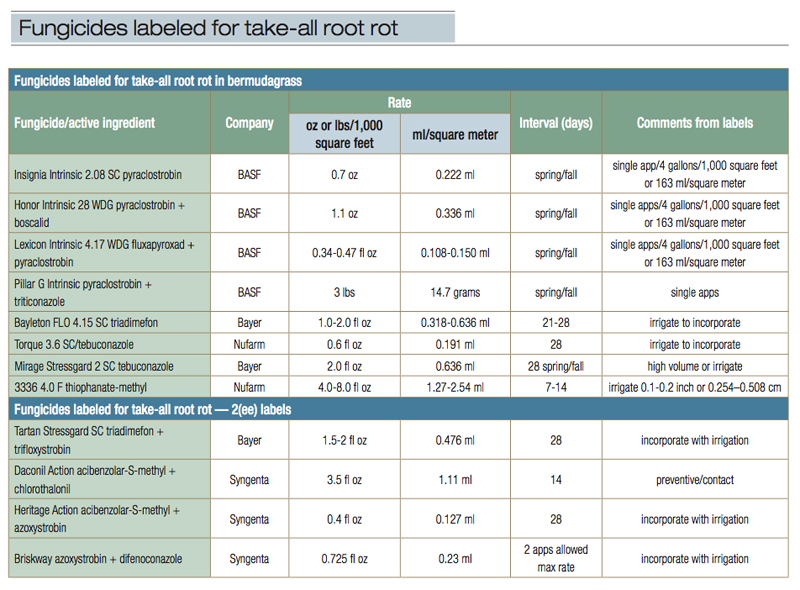
Abbreviations: oz, ounce or ounces; fl oz, fluid ounce or ounces; lbs, pounds; ml, milliliters.
Note. Section 2(ee) of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and General Pesticide Rule WAC 16-228-1225 allow pesticide users to apply pesticides under certain limited conditions that are not specified on a pesticide label.
Table 1. Fungicides labeled for take-all root rot in bermudagrass, and fungicides labeled for take-all root rot with 2(ee) labels.
The observation of field symptoms, site characteristics, weather conditions before and during disease occurrence, and microscopic examination of ERI, root rot and presence of lobed hyphopodia generally serves as the diagnosis of take-all root rot by most labs. As time goes on and pathologists begin to use molecular methods more commonly in routine diagnostics, as they do now in research projects, diagnosis will become more precise.
Disease triangle
The previous discussion of purported causal agents was necessary to introduce the complexity of root-rot pathogens that can be present in bermudagrass root systems. Monica Elliott, Ph.D., of the University of Florida, who first described and reported extensively on bermudagrass decline, also noted many years ago that bermudagrass is commonly colonized with GGG. It is not unusual to detect GGG with bermudagrass that appears healthy and functions adequately. In fact, GGG is considered less virulent among the species of Gaeumannomyces than GGA or GGT.
Typically, as with most diseases, the events that promote overt disease are environmental, coupled with an innate susceptibility of bermudagrass cultivars used on putting greens. Environmental factors include weather, soil and water characteristics and management factors. Put simply, we typically see take-all root rot after abundant rains or persistent cloudy weather. Low fertility, very low cutting heights, high soil and water pH, and, perhaps, low availability of manganese are other factors that increase the potential for severe disease. In bermudagrass, take-all root rot is most severe on putting greens, and higher heights of cut of the same cultivar on green collars ordinarily do not exhibit severe symptoms.
Epidemics in the Carolinas
In the Carolinas, we have had back-to-back fall tropical systems that have likely kicked off the epidemics of take-all root rot. In the fall of 2015, Hurricane Joaquin killed 33 crew members from the American cargo ship El Faro, and later provided moisture to another system that caused extreme flooding in South Carolina, with several deaths in Columbia, S.C. Hurricane Matthew occurred in October 2016, flooding much of eastern North Carolina and South Carolina. Both systems were devastating storms, and in addition to the tragic losses of life and property, they provided abundant water and persistent cloudy weather that weakened bermudagrass.
These storms occurred at a time of year when days are shorter and night temperatures are cooler than 65 F (18.3 C), slowing the growth of bermudagrass every year and limiting its ability to grow out of symptoms. With the storms, overt diseases such as Pythium blight initially occurred on bermudagrass as a result of the abundant rains. Underground, however, roots infected with GGG further weakened bermudagrass, and basketball-sized patches occurred a month or so after the storm systems had passed. Persistent cloudy, wet weather and cold temperatures caused these patches to persist for months through the winter and spring of 2015-2016. After Hurricane Matthew, the weather turned sunny and dry for several months, and symptoms did not persist until cloudy, wet weather again occurred in December 2016 and January 2017. We have seen the most widespread take-all root-rot disease in the Carolinas during the past two years, but we certainly see some disease every year, typically in the eastern and southern regions, where rains and cloudy weather are more frequent.
Management
Management of take-all root rot is difficult, which is generally true of all root diseases. Bermudagrass has little known resistance to the disease, and root systems of greens-type bermudagrasses are not extensive, especially when they are cultured in sand-based root-zone mixes and are maintained at consistently low cutting heights. Nevertheless, when weather patterns induce chronic stress from rain and cloudy weather, practices that will lessen the impacts of disease are: raising cutting heights, becoming less aggressive with verticutting depth, and using rolling for better putting speed and consistency. General recommendations also include using acidifying fertilizers, such as ammonium sulfate, to lower rhizosphere pH and allow micronutrients such as manganese to be more plant-available. Adequate manganese concentration and availability is also suggested, although research with bermudagrass showing suppression of take-all root rot with manganese is lacking; manganese has been demonstrated to influence take-all of wheat (GGT) and take-all patch in creeping bentgrass (Agrostis stolonifera) (GGA).

Take-all root rot symptoms in January 2017 after fall fungicide treatments showed good control with Lexicon Intrinsic, Mirage Stressgard, Tartan Stressgard and an experimental fungicide. Xzemplar (fluxapyroxad, BASF) and Exteris Stressgard (fluopyram + trifloxystrobin, Bayer) (not shown) gave poor results.
We do not have soil temperature targets for initiation of fungicide applications as we do with take-all patch in bentgrasses or summer patch in Poa annua. Therefore, being aware of and tuned in to weather patterns that are harmful to bermudagrass can assist superintendents in being proactive with management adjustments and fungicide applications. Take-all root rot occurs in every environment where bermudagrass is cultured, so fungicide use and timing likely depend on many factors that are appropriate in certain habitats but less important in others.
Timing of fungicidal control
Optimal timing of fungicide applications requires consideration of environmental conditions that favor infection, the relative health of the host, and the potential of bermudagrass to sustain severe damage from take-all root rot. With these considerations, the disease outbreaks in the Carolinas probably could not have happened at a worse time for the transition zone. As mentioned already, bermudagrass naturally slows its growth rate in fall. Also, bermudagrass will initiate new root growth on young stolons and rhizomes in fall, winter and spring. As with spring dead spot, severely infected roots are less likely to survive the winter dormancy period. For these reasons, late summer into fall may be a good time to consider applying fungicides with known activity for take-all root rot in transition zone environments; in tropical and subtropical environments, fungicides are generally applied before and during rainy season periods. In South Florida, fungicides for take-all root rot may be applied in summer and into fall, while in the Carolinas, preventive treatments may not begin until late summer or early fall. Depending on fungicides used, other diseases can also be controlled to a degree, including dollar spot, leaf spot, spring dead spot and fairy ring.
Products
Table 1 lists fungicides with labels that specifically list Gaeumannomyces graminis var. graminis, take-all root rot or bermudagrass decline on the label. Labels that mention take-all patch (caused by GGA), spring dead spot (even when GGG is listed), summer patch or necrotic ring spot are not listed, as these are bona fide different diseases. Through trials and experience, researchers have found that fungicides that are good for a particular root-rot disease may or may not be effective for another, regardless of how similar the diseases appear to be. Therefore, this list is conservative and does not include some fungicides where there is likely good activity. Further testing and clarification of ambiguous labels will assist with choices in the future.
Trials, 2016
In the summer and fall of 2016, I had the opportunity to test some fungicides for control of take-all root rot. The trials were conducted on a MiniVerde bermudagrass turf nursery on a golf course in coastal South Carolina with a history of severe take-all root rot. Two experiments were conducted.
Experiment 1
The first experiment was designed to look at timing of fungicides to determine whether summer applications might prevent disease outbreaks in fall and winter. Three timings were evaluated with fungicides that were considered standards labeled for take-all root rot (Table 1). These included 3336 F (thiophanate-methyl) at 4 fluid ounces, Bayleton FLO SC (triadimefon) at 1 fluid ounce, Insignia Intrinsic (pyraclostrobin) at 0.7 fluid ounce, and Tartan Stressgard (triadimefon + trifloxystrobin) at 2 fluid ounces/1,000 square feet. Each fungicide was applied three times, 21 days apart, with an early, mid- and later application timing. Timing 1 dates were: July 13, Aug. 3 and Aug. 24. Timing 2 dates were delayed 21 days: Aug. 3, Aug. 24 and Sept. 14. Timing 3 dates were delayed 42 days from initiation: Aug. 24, Sept. 14 and Oct. 5. The later timing, beginning in late August and ending with the third application in early October, was the only treatment that prevented take-all symptoms when they recurred in December and January (data not shown), and disease was reduced only by Tartan Stressgard or Insignia Intrinsic in that trial. No effects were seen from Bayleton or 3336 F for take-all root rot control.
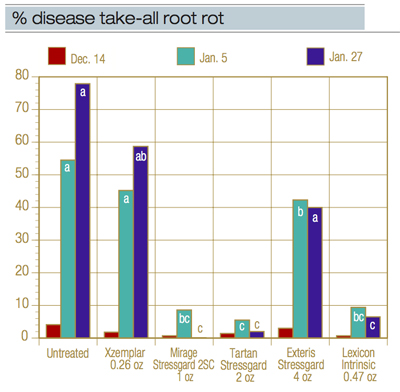
Figure 1. Take-all root rot disease severity after preventive fungicides were applied Oct. 18 and Nov. 15, 2016. Bars within a rating date are not significantly different if they are labeled with the same letter. Products tested are: Xzemplar (fluxapyroxad, BASF), Mirage Stressgard, Tartan Stressgard, Exteris Stressgard (fluopyram + trifloxystrobin, Bayer) and Lexicon Intrinsic. No significant differences were seen among treatments on Dec. 14.
This result does not mean those fungicides are ineffective, but simply that in this particular experiment, they may have been deployed at the wrong time (too early). It also showed that at least two fungicides, Insignia Intrinsic and Tartan Stressgard, did provide some control (about 60% suppression of symptoms compared with the untreated control), indicating efficacy from these products when applied in late summer into fall, with three applications spaced 21 days apart.
Experiment 2
The second experiment was designed to evaluate some newer fungicides or fungicides previously not labeled for preventive control of take-all root rot, and these were deployed only in the fall. They were sprayed only twice: on Oct. 18 and Nov. 15, 2016. Before the application of the experimental fungicides in fall, the space on the turf nursery had been exposed to a preventive fungicide program, which was discontinued about three weeks before initiation of the second trial. In both experiments, the fungicides were sprayed at 2.1 gallons/1,000 square feet (85.56 milliliters/square meter) and then incorporated into the root zone with overhead irrigation.
Figure 1 lists the fungicides, rates and dates of application in experiment 2, which looked at newer fungicides. We found that the SDHI fungicides Xzemplar and Exteris Stressgard showed very little effectiveness in this trial, but that Mirage Stressgard, Tartan Stressgard, Lexicon Intrinsic and an unlabeled experimental fungicide had excellent efficacy and largely controlled the symptoms nearly completely.
Conclusions
As expected, growth regulation occurred because of the triadimefon in Tartan Stressgard and the tebuconazole in Mirage Stressgard. However, the amount of growth regulation was acceptable because the materials were applied at reasonable rates for putting greens and the applications were a month apart. Nevertheless, one should be cautious with these materials because of their growth-regulating properties. We also saw excellent control from Lexicon Intrinsic and an experimental material. If Lexicon Intrinsic is used at this timing in the Carolinas, it will also augment spring dead spot control, for which it is very good. These fungicides will also assist in controlling dollar spot, leaf spot and fairy ring.
On the basis of both of these experiments, we have some data pointing us toward deployment in late summer and early fall to mid-fall applications. This does not mean these are the only times that fungicides may need to be applied for take-all root rot. Further experiments are needed to continue testing these and other materials to refine our recommendations and strategies for fungicide control of take-all root rot in bermudagrass putting greens for the Carolinas.
Acknowledgments
The author thanks Bayer Environmental Science, BASF and Nufarm for support in evaluating their products in this research.
References
- Elliott, M.L. 1991. Determination of an etiological agent of bermudagrass decline. Phytopathology 81:1380-1384.
- Elliott, M.L., E.A. Des Jardin and J.M. Henson. 1993. Use of a polymerase chain reaction assay to aid in identification of Gaeumannomyces graminis var. graminis from different grass hosts. Phytopathology 83:414-418.
- Fouly H.M., H.T. Wilkinson and L.L. Dormier. 1996. Use of random amplified polymorphic DNA (RAPD) for identification of Gaeumannomyces species. Soil Biology and Biochemistry 28:703-710.
- Freeman, J., and E. Ward. 2004. Gaeumannomyces graminis, the take-all fungus, and its relatives. Molecular Plant Pathology 5:235-252.
- Hernandez-Restrepo, M., J.Z. Groenewald, M.L. Elliott, G. Canning, V.E. McMillan and P.W. Crous. 2016. Take-all or nothing. Studies in Mycology 83:19-48.
- Smiley, R.W., P.H. Dernoeden and B.B. Clarke. 2005. Compendium of turfgrass diseases. 3rd edition. APS Press, St. Paul, Minn.
- Vines, P.L., M. Tomaso-Peterson and T. Allen. 2015. Evaluating novel ectotrophic root-infecting fungi as possible etiological agents for summer decline of ultradwarf bermudagrass. Phytopathology 105 (Suppl. 2) S2:11.
- Vines, P.L., M. Tomaso-Peterson, B.R. Stewart, T.W. Allen and F. Meyer. 2013. Identification of ectotrophic root-infecting fungi in association with ultradwarf bermudagrasses (Cynodon dactylon x transvaalensis). Phytopathology 103 (Suppl. 1) S1:10.
- Wong, P.T.W. 2002. Gaeumannomyces wongoonoo sp. nov., the cause of a patch disease of buffalo grass (St. Augustine grass). Mycological Research 106:857-862.
Bruce Martin is the research and Extension turfgrass pathologist for South Carolina and a professor at the Pee Dee Research and Education Center, Clemson University, Florence, S.C.