

Figure 1. Representative scanning electron microscopy images of hydrophobic sand (top) and a clean sand grain that has never been used. Scales in micrometers (10-6 meter) are on the lower right corner of each image. Note the layers of organic coatings on the sand grain at the top and the clean surface on the sand grain below it. The hydrophobic sand grain was collected from a 7-year-old USGA green where localized dry spot has been documented. The hydrophobicity level was determined to be severe based on the water droplet penetration test, which had a result of >2,500 seconds. Photos by Enzhan Song
Editor’s note: This research was funded in part by the United States Golf Association.
Soil water repellency, also referred to as soil hydrophobicity, slows water infiltration into the soil profile and, in some cases, causes water to bypass hydrophobic areas, ultimately leading to localized dry spot (LDS). This undesirable soil condition is a widespread issue, but it is more common on sand-based growing media such as USGA greens, because the specific surface area of sand particles is much smaller than that of other soil minerals (7).
Wetting agents and hydrophobicity
Soil hydrophobicity is believed to be caused by the formation of complex organic acids, such as humic acid and fulvic acid, which coat the surface of sand particles (Figure 1). These organic acids are formed during the natural decomposition of soil organic matter over time (14).
Wetting agents that contain both hydrophobic (oil-loving) and hydrophilic (water-loving) groups in their molecules are the primary management tool for controlling soil water repellency. The desire for control of soil hydrophobicity and improvement of soil water retention has led to a widespread use of wetting agents in the turf industry. A 2006 survey found that 98% of golf course superintendents who responded had experience with wetting agents (5).
Within the scientific community, there is also a high level of interest among turf researchers, as evidenced by the number of publications on this topic. As of Aug. 21, 2018, a search of the term “wetting agent” in the Turfgrass Information File produced 1,438 records.
Despite the wide use of and interest in wetting agents in our turf community, many questions have yet to be answered (5, 7-11). One of the questions brought up by Keith Karnok, Ph.D. — one of the foremost authorities on soil water repellency (1) — concerns whether certain wetting agents can live up to the advertising and remove organic coatings from the soil particles (9). Removal of the organic coatings can ultimately reverse the soil’s water-repellent condition and, consequently, could allow the turf manager to move away from regular application of wetting agents throughout the growing season — until organic acids accumulate on the sand surface again.
Theoretically, reversal of water repellency is possible, because humic acids are soluble in high-pH solutions such as sodium hydroxide (NaOH), and fulvic acid can be dissolved in both acidic and basic solutions.
A field experiment on a USGA green found that sequential applications of NaOH at 0.1 M over three days removed substantial amounts of humic substances and reduced soil hydrophobicity levels from strong to moderate (6). However, application of NaOH increased soil pH from 5.9 to 8.3 and resulted in variable levels of phytotoxicity on creeping bentgrass (Agrostis stolonifera L.).
Another approach is to use wax-dependent metabolizing bacteria such as Streptomyces species (13), Rhodococcus species (12) and Mycobacterium species (2). However, this bioremediation approach was discovered to be time-consuming, and results were inconsistent depending on the environmental conditions.
Alternatively, some wetting agents with strong amphiphilic properties have been found to be capable of concentrating and extracting hydrophobic organic compounds from water in the laboratory (4). In the turf market, some commercial compounds claim to remove organic coatings from the hydrophobic sand surface. For example, a product called OARS (organic acid removal system; Aqua-Aid), which (based on the label information) contains 80% polyoxyalkylene polymers and 10% potassium salt of alkyl substituted maleic acid, is being promoted for such a purpose. Until this study, however, there was no research-based evidence to evaluate such an effect. Therefore, the objective of this research was to investigate whether selected wetting agents could remove hydrophobic organic coatings from sand surfaces.
Materials and methods
Sand was collected from areas where localized dry spot had been documented on a 7-year-old USGA green at the Turf Research Facility at the University of Missouri in Columbia, Mo. The degree of hydrophobicity was determined to be moderate based on the molarity of ethanol droplet test. Once bench-dried, sand was separated from plant debris before homogenization and packed into PVC columns.
After being packed to uniformity, all columns contained the same amount of sand with a uniform bulk density and porosity, which was equivalent to a pore volume of 2 ounces (58 milliliters). Wetting agents applied included Matador (ENP Investments) and OARS and a surfactant named pHAcid (Numerator Technologies), as well as distilled, deionized water as a control.
All three wetting agents were mixed with water at the highest label-suggested rates and applied to the sand columns at 2.36 ounces (70 milliliters), which was greater than the pore volume and ensured complete saturation of the sand columns. After 24 hours, sand columns were then washed at the pore volume of 2 ounces of water three times at 30-minute intervals (Figure 2, below).
Figure 2. Flow chart representing application of wetting agents and three sequential washes.
All leachates collected from application of the wetting agents and the sequential washes were acidified using sulfuric acid to remove inorganic carbons. The organic carbons in the soluble forms in the leachates (termed dissolved organic carbon) and in the insoluble forms in the leachates (termed particulate organic carbon) were then determined. The underlying rationale for this measurement is that all organic compounds contain carbon. Therefore, determining the organic carbon in the leachates is an indirect measurement for the amount of organic compounds that could be potentially washed off from the hydrophobic sands.
The amount of dissolved organic carbon was quantified by using a Shimadzu TOC-VWP analyzer equipped with an autosampler ASI-V. The particulate organic carbon was evaluated by first separating the particulate organic matter from the leachates using a centrifuge before determining the mass of the particulate organic matter via combustion.
Solid phase organic carbon in the sand profiles before and after treatment was determined by using a LECO TruSpec CN carbon/nitrogen analyzer (LECO Corp.). The effect of treatments on reversing the water-repellent condition of the sands was also determined using the molarity of ethanol droplet test.
All treatments were arranged in a completely randomized design with three replications, and the entire experiment was repeated once. Collected data were analyzed, and no treatment-by-experiment interaction was detected for all response variables measured. Therefore, data from the two experiments were pooled, and significant means were separated.
Results and discussion
After treatment application and the three sequential washes, total dissolved organic carbon and particulate organic carbon present in all leachates of each sand column were summarized in Figure 3. The water-only treatment removed a minimal amount of dissolved organic carbon, indicating a small portion of the organic compounds in the sand profile was water-soluble (Figure 3A). Compared with water-treated columns, the same amount of dissolved organic carbon was removed from columns treated with pHAcid, suggesting that the effect of pHAcid was similar to that of water.
In contrast, Matador and OARS both removed a substantially greater amount of dissolved organic carbon than water did. It is important to keep in mind that applications of Matador introduced 1,765 mg of dissolved organic carbon, and applications of OARS introduced 1,409 mg, as the wetting agents are also organic compounds in a water-soluble form. In contrast, application of pHAcid added only 23 mg of dissolved organic carbon.
Collectively, it appeared that the amount of dissolved organic carbon found in leachates from Matador- and OARS-treated sand columns accounted for 91% and 51% of the dissolved organic carbon introduced by the two treatments, respectively. Although we were not able to precisely separate the indigenous dissolved organic carbon introduced by the treatment from the dissolved organic carbon that could be washed out from the sand profile (even with water as a control), it is clear that at least half of the dissolved organic carbon introduced by OARS was retained in the sand system despite three washes, suggesting a strong sorption between OARS and the sand.
In terms of insoluble particulate organic carbon, the water-only treatment removed a minimal amount of particulate organic carbon, likely because the sand collection and handling process might have weakened the attachment between the organic coatings and sand. OARS or pHAcid removed the same amount of particulate organic carbon from sand columns as the water control (Figure 3B).
Treatment with Matador, however, removed 6.9 times the amount of particulate organic carbon as water alone. Based on the type of filter we used in this experiment, the particles we collected to determine the particulate organic matter were finer than 0.05 mm, which classifies the organic carbon we removed as fine particulate organic carbon.
It has been reported that, even when present at a low concentration, fine particulate organic carbon significantly increases soil water repellency (3). Therefore, our results suggest that application of Matador followed by three sequential washes removed a substantial amount of water-insoluble organic coatings from the sand columns, which likely contributes to an improvement in wettability of the sand.
The solid phase organic carbon in the water-only treatment remained statistically constant compared with the amount of solid phase organic carbon found in the same source of sand that was not treated. Sands treated with pHAcid showed a 16% reduction in solid phase organic carbon compared with the sands that were not treated. Compared with the untreated sands, those treated with Matador resulted in the same amount of solid phase organic carbon, while application of OARS produced 27% greater solid phase organic carbon.
This intriguing result suggests that application of OARS introduced an additional source of organic compounds to the sand profile, and these organic compounds remained in the sand system in a solid form. This result again suggests a strong sorption of the OARS molecules to the sand, corroborating the findings on the dissolved organic carbon as described above.
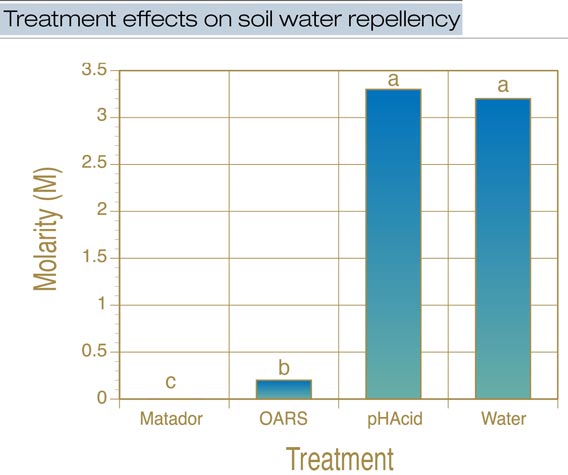
Figure 4. Effect of treating soil-repellent sand with one of two wetting agents, a surfactant or deionized water, followed by three sequential washes. Soil water repellency was determined by using the molarity of ethanol droplet test. Bars labeled with the same letters are not significantly different.
After application of treatments and three washes, sand treated with pHAcid showed no change in its hydrophobicity compared with the water-only treatment (Figure 4, above). In contrast, application of OARS significantly reduced the hydrophobicity of the sand to the category of low water repellency. Application of Matador completely reversed the hydrophobicity of the sand and resulted in a wettable sand.
Conclusion
Collectively, results from this research indicate that both OARS and Matador are promising for reducing soil water repellency to a minimum (OARS) or zero (Matador), following one application and three sequential washes.
Although the reasons are not fully understood, our data suggest that OARS molecules exhibit strong sorption to the hydrophobic sand surfaces, and a significant amount of the molecules likely remain in the sand system despite sequential washes. In comparison, applying Matador may wash out a greater amount of organic compounds from the system in both water-soluble and insoluble forms.
Our results also suggest that deep irrigation following application of wetting agents such as Matador likely increases removal of organic coatings from the sand profile. However, caution is needed, as this is strictly a laboratory-based experiment, and implications for field conditions could be complicated as plants, soil and microorganisms interact.
The research team at the University of Missouri has been performing more in-depth laboratory and field experiments in this line of research, so stay tuned for more research findings.
Acknowledgments
This material is based on work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 1006256 to principal investigator Xi Xiong. The authors would also like to thank the United States Golf Association for funding.
The research says ...
- Soil water repellency leads to localized dry spot, a widespread issue that is more common on sand-based growing media such as USGA greens.
- In a laboratory setting, sand collected from a green with localized dry spot was treated with two wetting agents, a surfactant and deionized water.
- Sand treated with either deionized water or the surfactant showed no change in soil water repellency.
- The wetting agents OARS and Matador showed promise for reducing soil water repellency to minimum or none. Matador removed a significant portion of organic coatings from the sand, suggesting that it can reverse soil water repellency.
- Because this experiment took place in a lab, caution is needed when interpreting the results for field conditions.
Literature cited
- Dekker, L.W., K. Oostindie and C.J. Ritsema. 2005. Exponential increase of publications related to soil water repellency. Australian Journal of Soil Research 43:403-441.
- Dunkelberg, E., U. Boeckelmann, B. Braun, D. Diehl, G.E. Schaumann and E. Grohmann. 2006. Do wax-degrading bacteria influence water repellency of soil? In: First International Biohydrology Conference, Prague, Czech Republic, Sept. 20-22, 2006.
- Franco, C.M.M., M.E. Tate and J.M. Oades. 1995. Studies on non-wetting sands. I. The role of intrinsic particulate organic-matter in the development of water-repellency in non-wetting sands. Australian Journal of Soil Research 33:253-263.
- Frankewich, R.P., and W.L. Hinze. 1994. Evaluation and optimization of the factors affecting nonionic surfactant-mediated phase separations. Analytical Chemistry 66:944-954.
- Karnok, K.J. 2006. Which wetting agent is best? Golf Course Management 74(7):82-83.
- Karnok, K.A., E.J. Rowland and K.H. Tan. 1993. High pH treatments and the alleviation of soil hydrophobicity on golf greens. Agronomy Journal 85:983-986.
- Karnok, K.J., and K.A. Tucker. 1989. The cause and control of localized dry spots on bentgrass greens. Golf Course Management 57(8):28-34.
- Karnok, K.J., and K.A. Tucker. 2000. FAQs about LDS: Localized dry spot fuels superintendents’ questions. Golf Course Management 68(6):75-78.
- Karnok, K.J., and K.A. Tucker. 2002. Water-repellent soils, Part 2: More questions and answers. Golf Course Management 70(7):49-52.
- Karnok, K.J., and K.A. Tucker. 2007. More FAQs about LDS: Hot spots and label rates. Golf Course Management 75(6):109-111.
- Karnok, K.J., and K.A. Tucker. 2009. Answers to questions about wetting agents. Golf Course Management 77(8):82-85.
- McKenna, F., K.A. El-Tarabily, S. Petrie, C. Chen and B. Dell. 2002. Application of actinomycetes to soil to ameliorate water repellency. Letters in Applied Microbiology 35:107-112.
- Roper, M.M. 2004. The isolation and characterization of bacteria with the potential to degrade waxes that cause water repellency in sandy soils. Australian Journal of Soil Research 42:427-434.
- Tucker, K.A., K.J. Karnok, D.E. Radcliffe, G. Landry Jr., R.W. Roncadori and K.H. Tan. 1990. Localized dry spots as caused by hydrophobic sands on bentgrass greens. Agronomy Journal 82:549-555.
Enzhan Song was a Ph.D. student at the University of Missouri, Columbia, and is currently with Agriculture Development Group Inc. in Eltopia, Wash. All other authors are affiliated with the University of Missouri, Columbia, Mo.: Keith W. Goyne is associate director of the School of Natural Resources and professor of environmental soil chemistry; Robert J. Kremer is an adjunct professor of soil science in the Division of Plant Sciences and in the Department of Soil, Environmental & Atmospheric Sciences; Stephen H. Anderson is the William A. Albrecht Distinguished Professor of Soil and Environmental Sciences and an adjunct professor in Plant Sciences; and Xi Xiong is an associate professor in the Division of Plant Sciences.