
Pressure chamber systems used for water retention measurement. Photo by Stephen Anderson
Editor’s note: This research was funded in part by GCSAA through the Environmental Institute for Golf.
For many of us, wetting agents are common tools we use for the purposes of conserving irrigation water and/or managing soil water repellency. It has been estimated that, on average, the U.S. golf industry uses more than 2 billion gallons of irrigation water on a daily basis, and wetting agents are the No. 1 measure adopted by 92% of golf facilities for water management (18).
On golf courses, especially on sand-based growing mediums such as USGA greens, soil water repellency is, unfortunately, an inevitable condition. Although soil water repellency can occur on all soil types, coarser particles, such as the sands used to build and/or topdress greens, are more susceptible to the development of water repellency because they have a significantly smaller (>105 times) specific surface area (area/mass) compared with peat and clay (3).
It is also argued that the significantly smaller specific surface area, along with the higher distribution of macropores found in sandy soils, provides a preferred habitat for fungal growth rather than bacteria, further promoting the development of soil water repellency (8). Consequently, water bypasses repellent areas and forms preferential flow, leading to the development of localized dry spot. Regardless of management intensity, water repellency will eventually develop, and localized dry spot may be observed on sand-based greens as soon as six months after construction (19).
Water repellency, or hydrophobicity, is a phenomenon in which a surface repels water into individual droplets, thus inhibiting water infiltration into porous mediums such as soil. This repelling force is caused by strong cohesion within the water body, which is termed “surface tension” or “surface-free energy.”
Under normal conditions (68 F; 20 C), surface tension of water is 72.75 millinewtons/meter (15). Surfaces with a surface-free energy greater than 72.75 millinewtons/meter possess stronger adhesive forces than the cohesive force within water, which forces attraction with contacting water molecules and spreads them over the surfaces, sequentially retaining water and forming hydrophilic surfaces.
When a soil medium becomes hydrophobic because of an accumulation of organic acids, its surface-free energy decreases to below 72.75 millinewtons/meter, resulting in a failed attraction to bypass water molecules and, inevitably, the development of water repellency. Addition of wetting agents — amphiphilic molecules with hydrophobic tails and hydrophilic heads in their chemical structures — reduces the surface tension of water and simultaneously allows the wetting agents to pass into the soil, thereby increasing infiltration and water retention (17).
Since the development of the first patented wetting agent product — a blend of nonionic surfactants such as alkylphenol ethoxylate (6) — and the early research that investigated soil water repellency on turf in the 1960s (13), tremendous advances have been made in various wetting agents that include formulations with ethylene oxide and propylene oxide block copolymers (1, 2, 7), for the purposes of increasing water infiltration, promoting homogeneous soil moisture and, if possible, preventing the development of soil water repellency. Performance of those wetting agents, however, is dramatically influenced by formulations, application rates and environmental conditions (5, 12). This article, therefore, aims to summarize some of our recent research findings regarding inherent differences among wetting agents and, we hope, provide guidance in selecting the appropriate wetting agents to meet various goals.
Effect of wetting agents on surface tension of water
As described above, adding wetting agents can reduce the surface tension of water, allowing the wetting agents to enter the hydrophobic soil medium and thereby facilitate infiltration. In this laboratory-based experiment, 15 commonly used wetting agents in the turf market (see the 15 products in Table 1) were mixed with water at five rates — 4, 2, 1, 0.5 and 0.25 times the rates suggested on the label — before determining their surface tension using the Attention Theta Lite tensiometer (Biolin Scientific). This experiment was designed as a completely randomized design with a factorial combination of wetting agents and various rates, with three replications and two repeats.
A wide range of surface tension was seen among the 15 selected wetting agents (Figure 1, below). The highest surface tension found was 44.8 millinewtons/meter at label rate, produced by InfilTRx. The lowest surface tension was 22.0 millinewtons/meter, which was caused by applying Break-thru S240 at the label rate and was less than half the surface tension from InfilTRx. For reference, the surface tension of tap water in our laboratory was 72.8 millinewtons/meter.
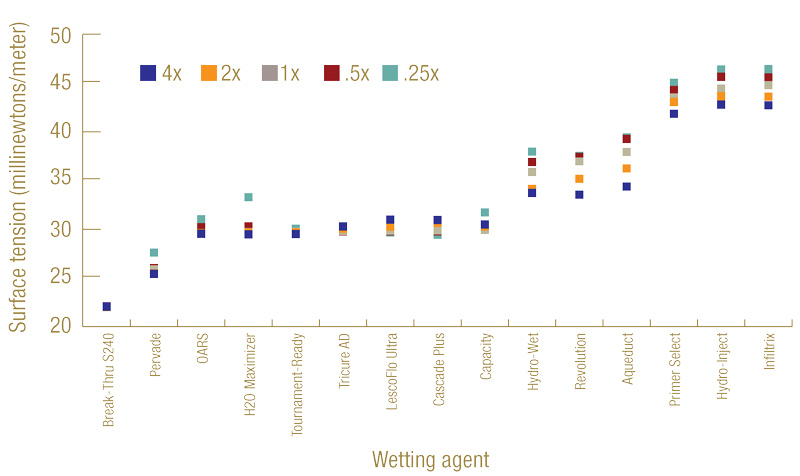
Figure 1. Distribution of surface tension (millinewtons/meter) of 15 wetting agents at 4, 2, 1, 0.5 and 0.25 times the label suggested rates.
These results suggested that all wetting agents tested, regardless of rates, substantially reduced the surface tension of water, indicating improved infiltration into a hydrophobic soil surface. We also saw a trend of lower surface tension generally being associated with higher concentrations of the same product. Our early research found a negatively correlated relationship between surface tension and hydraulic conductivity for a given wetting agent solution (17). Therefore, a wetting agent that produces relatively higher surface tension is likely to produce relatively lower infiltration into hydrophobic soil.
Effect of wetting agents on water infiltration
In the laboratory, six wetting agents were then tested for their effect on water infiltration into hydrophobic sands. The wetting agents selected were H2O Maximizer (KALO), TriCure AD (Mitchell Products), Capacity (Becker Underwood), Aqueduct (Aquatrols), Primer Select (Aquatrols) and InfilTRx (Aquatrols).
Sands that meet the USGA recommendation for green construction were treated with octadecylamine in the laboratory to create steady hydrophobicity at 7.2 molar (based on the molarity of ethanol droplet test) (11). This level of hydrophobicity is categorized as severely water-repellent (10). The sand was then packed uniformly in an infiltration system made of PVC pipes, and the infiltration of the selected wetting agents at their label-suggested rates was determined with the method described in our earlier publication (17). This experiment was a completely randomized design with three replications and one repeat.
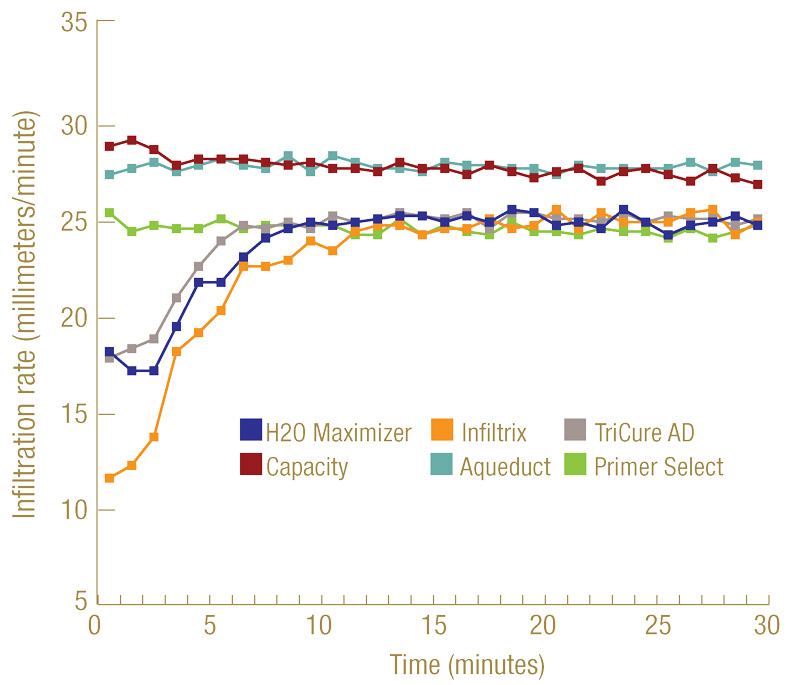
Figure 2. Infiltration of six selected wetting agents into hydrophobic sand. The water-only treatment did not penetrate into the hydrophobic sand, hence its infiltration was not generated.
All wetting agents were tested at their highest label-suggested rates. Within 30 minutes after application and by 10 minutes into ponding, all the selected wetting agents reached a steady infiltration rate (Figure 2, above). The steady infiltration rates of Capacity and Aqueduct reached 1.06 inch (27 millimeters)/minute, while the other four products maintained a steady rate of 0.94 to 0.98 inch (24 to 25 millimeters)/minute. All the rates surpassed the minimum saturated hydraulic conductivity of 0.1 inch (2.5 millimeters)/minute recommended by the USGA for green construction. Under the testing conditions, tap water alone was not able to penetrate into this severely hydrophobic sand, hence no infiltration data are presented.
Effect of wetting agents on water retention
Water retention of a soil medium is highly influenced by soil physical and chemical properties, such as texture, porosity and organic matter content. When a wetting agent solution is introduced to a hydrophobic soil, its influence on soil water retention becomes a function of its chemical properties, specifically the balance between the hydrophobic and lipophilic regions, which is called the hydrophilic-lipophilic balance (16).
In this experiment, we first collected sands from a USGA green where localized dry spots had been documented. After removing plant debris, the sands were thoroughly mixed, bench-dried and stored for future use. Using the molarity of ethanol droplet test, we determined that the hydrophobicity of the sand was 3.4 molar, which is considered very severe (11). The organic matter of the sand was determined to be 1.73%. The particle analysis revealed that the sands consisted of 6.76% very coarse sand, 12.74% coarse sand, 58.73% medium sand, 20.75% fine sand and 1.02% very fine sand. At a bulk density of 0.86 ounce/cubic inch (1.49 grams/cubic centimeter), the total porosity of the sands was 43.9%, with 24.5% capillary porosity and 19.4% air-filled porosity.
The hydrophobic sands were then used in a pressure chamber experiment using porous plates containing sands that had previously been saturated with the 15 wetting agent solutions described above at the highest label rates. The pre-treated sands were subjected to pressure treatment at -0.42 psi (-2.9 kPa) for estimated field capacity and at -217.56 psi (-1,500 kPa) for permanent wilting point (14). For perspective, 1,500 kPa is equivalent to 15 times atmospheric pressure at sea level. After equilibration, the gravimetric water content corresponding to each pressure value was calculated and adjusted to volumetric water content. Plant-available water was then estimated by subtracting the lower limit (-217.56 psi, permanent wilting point) from the upper limit (-0.42 psi, field capacity) (4, 9). This experiment was arranged in a completely randomized design with four replications and one repeat.
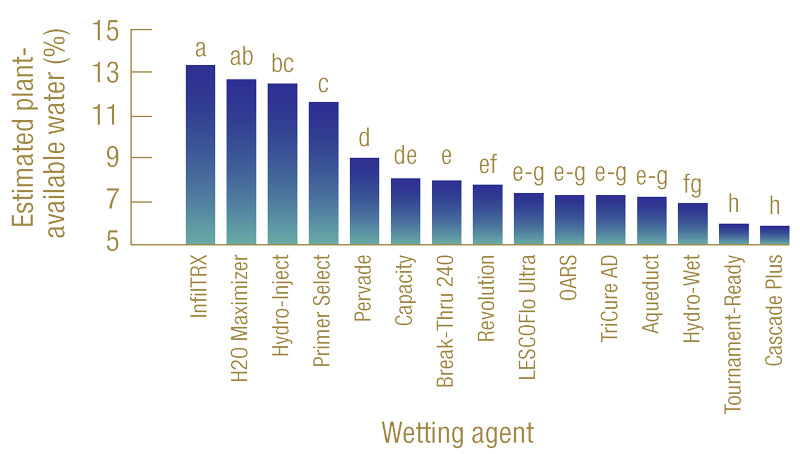
Figure 3. Estimated plant-available water (%) of 15 wetting agents evaluated. Bars labeled with different letters are significantly different from one another.
Results showed that the 15 wetting agents evaluated resulted in a broad range of plant-available water — between 13.3% and 5.5% (Figure 3, above). Without a wetting agent application, tap water alone failed to saturate the sand profile because of the nature of hydrophobicity. Based on the calculated plant-available water, the 15 wetting agents tested can be divided into three groups. The first group contains InfilTRx, H2O Maximizer, Hydro-Inject and Primer Select, with their plant-available water ranging roughly between 11% and 13%. The second group contains wetting agents that produced moderate amounts of plant-available water, ranging from 7% to 9%, and the last group includes Hydro-Wet, Tournament-Ready and Cascade Plus, with relatively low plant-available water of 6.5% or below.
It is important to emphasize that these results were based on the particular soil used in this experiment. Soils with different physical and chemical properties will most likely generate different amounts of water retention for a given wetting agent. Although not all wetting agents tested here are designed for water retention, our results also suggest that wetting agents vary substantially in their potential for retaining plant-available water and, therefore, for use in water conservation.
Conclusion
This article summarizes a few updates from our recent research on wetting agents and provides a glimpse of inherent differences among wetting agents because of their chemical properties. We believe we have provided strong evidence that not all wetting agents are created equal, and users should select the proper wetting agents discriminately based on their specific goals.
Funding
This material is based upon work that is partially supported by GCSAA and the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award No. 1006256.
The research says ...
- Significant differences were seen among the 15 wetting agents tested, but, regardless of rates, all substantially reduced the surface tension of water, indicating improved infiltration into a hydrophobic soil surface.
- All six wetting agents tested for their rate of water infiltration in hydrophobic sand surpassed the minimum saturated hydraulic conductivity rate of 0.1 inch/minute.
- The 15 wetting agents evaluated produced 13.3% to 5.5% plant-available water in hydrophobic sandy soil.
- Wetting agents differ in their effect on water surface tension and water infiltration and retention. Golf course superintendents should consider these differences when selecting a product.
Literature cited
- Alexandridis, P., and T.A. Hatton. 1995. Poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling. Colloids and Surfaces A: Physicochemical and Engineering Aspects 96(1): 1-46.
- Bates, F.S., and G.H. Fredrickson. 1990. Block copolymer thermodynamics: theory and experiment. Annual Review of Physical Chemistry 41(1):525-557.
- Blackwell, P.S. 1993. Improving sustainable production from water repellent sands. Western Australia Journal of Agriculture 34:160-167.
- Brady, N.C., and R.R. Weil. 1996. Soil water: characteristics and behavior. Pages 171-212. In: N.C. Brady and R.R. Weil. 11th edition. The nature and properties of soils. Prentice-Hall, Upper Saddle River, N.J.
- Cisar, J.L., K.E. Williams, H.E. Vivas and J.J. Haydu. 2000. The occurrence and alleviation by surfactants of soil-water repellency on sand-based turfgrass systems. Journal of Hydrology 231:352-358.
- De Boodt, M. 1972. Improvement of soil structure by chemical means. Pages 43-45. In: D. Hillel, editor. Optimizing the soil physical environment toward greater crop yields. Academic Press, New York.
- Green, R.J., S. Tasker, J. Davies, M.C. Davies, C.T. Roberts and S.J.B. Tendler. 1997. Adsorption of PEO-PPO-PEO triblock copolymers at the solid/liquid interface: A surface plasmon resonance study. Langmuir 13(24):6510-6515.
- Hallett, P.D., K. Ritz and R.E. Wheatley. 2001. Microbial derived water repellency in golf course soil. International Turfgrass Society Research Journal 9:518-524.
- Jiang, P., S.H. Anderson, N.R. Kitchen, K.A. Sudduth and E.J. Sadler. 2007. Estimating plant-available water capacity for claypan landscapes using apparent electrical conductivity. Soil Science Society of America Journal 71:1902-1908.
- Karnok, K.J., and K.A. Tucker. 2001. Wetting agent treated hydrophobic soil and its effect on color, quality and root growth of creeping bentgrass. International Turfgrass Society Research Journal 9:537-541.
- King, P.M. 1981. Comparison of methods for measuring severity of water repellence of sandy soils and assessment of some factors that affect measurement. Australian Journal of Soil Research 19:275-285. doi:10.1071/SR9810275
- Kostka, S.J., G. Schuermann and M. Franklin. 2007. Alkyl-capped block copolymer surfactants for remediation of soil water repellency and heterogeneous rootzone moisture. Journal of ASTM International 4:1-8.
- Morgan, W.C., J. Letey, S.J. Richards and N. Valoras. 1967. Using physical soil amendments, irrigation, and wetting agents in turfgrass management. California Agriculture 21(1):8-11.
- Ok, C.H., S.H. Anderson and E.H. Ervin. 2003. Amendments and construction systems for improving the performance of sand-based putting greens. Agronomy Journal 95:1583-1590.
- Parker, S.D. 1987. Encyclopedia of science and technology. McGraw-Hill, New York.
- Schick, M.J. 1987. Nonionic surfactants: Physical chemistry. 2nd edition. Dekker, New York.
- Song, E., J.G. Schneider, S.H. Anderson, K.W. Goyne and X. Xiong. 2014. Wetting agent influence on water infiltration into hydrophobic sand: II. Physical properties. Agronomy Journal 106:1879-1885. doi:10.2134/agronj14.0153
- Throssell, C.S., G.T. Lyman, M.E. Johnson, G.A. Stacey and C.D. Brown. 2009. Golf course environmental profile measures water use, source, cost, quality, and management and conservation strategies. Applied Turfgrass Science 6. doi:10.1094/ATS-2009-0129-01-RS
- Tucker, K.A., K.J. Karnok, D.E. Radcliffe, G. Landry, R.W. Roncadori and K.H. Tan. 1990. Localized dry spots as caused by hydrophobic sands on bentgrass greens. Agronomy Journal 82:549-555. doi:10.2134/agronj1990.00021962008200030023x
Xi Xiong is an associate professor in the Division of Plant Sciences, and Stephen H. Anderson is a William A. Albrecht Distinguished Professor of Soil and Environmental Sciences in the School of Natural Resources at the University of Missouri, Columbia, Mo.