
Figure 1. Algal crust on a creeping bentgrass putting green. Photos by John Inguagiato
Editor’s note: This research was funded in part by a grant to GCSAA from the Environmental Institute for Golf.
Cyanobacteria, or blue-green algae, are a common problem on putting greens. Several genera of cyanobacteria (for example, Lyngbya, Nostoc, Oscillatoria, Phormidium, etc.) are known to reduce putting green quality. Typically, cyanobacteria affecting putting greens are filamentous organisms that produce food through photosynthesis. They proliferate on putting greens under various conditions: where thin turf offers opportunities for colonization, where sunlight passes through the turf canopy to the soil surface, where frequent irrigation maintains surface moisture, and where nitrogen and phosphorous are readily available (1). Turf managers who use reclaimed water to irrigate putting greens have also reported increases in algae. Both increased use of reclaimed water and aggressive cultural practices that reduce the turf’s competitive ability promote opportunities for cyanobacteria to be a persistent challenge to the quality of putting greens.
Impacts of cyanobacteria on putting greens
Detrimental impacts of cyanobacteria on putting greens include the formation of algal surface crusts (1) and subsurface anaerobic conditions, both of which contribute, in part, to black layer. Surface crusts begin as films of actively growing algal colonies near the crown of turfgrass plants (Figure 1, above). During prolonged wet conditions, filaments grow upward along stem bases, extending vertically and horizontally to form a layer of cyanobacteria in the upper turf canopy that can smother small or weak tillers. Once dry, algae and matted tillers are reduced to a thin, impermeable crust in affected areas. As turf cover is reduced, water movement and growth of the turf through algal crusts is inhibited. Several species of cyanobacteria and green algae have been associated with the formation of algal crusts. More recently, yellow spot, a malady purportedly induced by cyanobacteria species, has been reported on putting greens in various regions of the United States (8). Symptoms are associated with aggregates of filamentous Phormidium species or Oscillatoria species, which are suspected of exuding compounds toxic to foliage.
Existing cyanobacteria control options
Control of algae on putting greens is accomplished primarily through cultural practices that promote rapid surface drying and favor vigorous turf growth (1). However, chemical control of cyanobacteria and/or green algae is often required on sites with shade and/or poor drainage where turfgrass growth has been reduced. Fungicides containing chlorothalonil, mancozeb, and copper hydroxide + mancozeb reduce surface algae symptoms. However, efficacy of these active ingredients in controlling algae has been variable. Label restrictions limit the total number of applications and/or the amount of chlorothalonil and copper hydroxide + mancozeb applied to putting surfaces each year, and some state and local restrictions prohibit chlorothalonil applications in environmentally sensitive areas. These restrictions can reduce the ability of turf managers to control algae when conditions favorable for algal growth are prolonged. Applications of copper hydroxide + mancozeb applied during elevated temperatures can also be phytotoxic to putting green turf, thus limiting its use during the summer.
Phosphites
Phosphite (H2PO3–) is a form of phosphorous that differs from phosphate (HPO4–) by the substitution of an oxygen atom for a hydrogen. Both forms, as well as the fungicide fosetyl-Al, are generally referred to as phosphonates. Phosphate is used by plants in various bioenergetic reactions and for structural cellular components. However, the hydrogen substitution in phosphite prevents the use of this ion as a phosphorous source for cellular activities and components in plants and other organisms (7). Phosphite fungicides and fertilizers are generally derived from a solution of phosphorous acid and water that has been pH-neutralized with a base, ester or alcohol (for example, potassium hydroxide or sodium hydroxide). The resulting products are commercially available formulations of potassium phosphite, ammonium phosphite or sodium phosphite. Phosphites have been demonstrated to suppress the severity of Pythium blight (caused by Pythium aphanidermatum) (2), anthracnose (caused by Colletotrichum cereale) (3), and Microdochium patch (caused by Microdochium nivale) (4).
Phosphites have also been observed to suppress algae. However, determination of the influence of phosphite source, application rate and efficacy relative to current chemical algae control products is needed. Therefore, the objectives of this study were to assess whether various phosphite sources could preventively suppress surface cyanobacteria colonization of putting green turf, and to determine optimal application rates of various phosphites for suppressing surface colonization by cyanobacteria.
Materials and methods
Site description
Field studies were conducted during 2010 and 2011 on an L-93 creeping bentgrass (Agrostis stolonifera) putting green established on a native sandy loam soil with a pH of 6.2 at the University of Connecticut Plant Science Research and Education Facility in Storrs, Conn. During the study, the field was mowed twice daily, five days per week, with a triplex mower adjusted to a bench setting of 0.125 inch (3.2 mm). Nitrogen was applied at moderate to low annual rates to reduce turf vigor and encourage algae infestation. Phosphorous and potassium levels were within the optimal range based on soil tests. Irrigation was applied at approximately 0.1 inch (2.5 mm), two to three times daily, and black-knit, 60% shade cloth was placed on the turf surface periodically to promote uniform colonization of the turf surface by cyanobacteria.
Experimental design and treatments
Two independent studies were conducted over two years within the same field, and were arranged as randomized complete block designs with four blocks.
Study 1: Phosphite sources. Study 1 consisted of 12 treatments: three commercial phosphite fungicides; three commercial phosphite fertilizers; potassium phosphite and potassium phosphate laboratory-prepared analytical standards; three non-phosphonate-based fungicides labeled for algae control; and a non-treated control. Rates of commercial products were generally selected based on the intermediate rate listed on product labels. Specific products and the active ingredient, phosphite source, formulation and application rate for each evaluated in Study 1 are listed in Table 1, Phosphite fungicides and fertilizers and contact fungicides. The phosphite fertilizer Magnum was evaluated only during 2011.
Study 2: Phosphonate formulation and rate. Treatments in Study 2 contained two main factors: phosphonate formulation and rate. Phosphonate formulations included a potassium phosphite fungicide (Alude, Cleary Chemical Corp.), potassium phosphite fertilizer (Phosphite 30, Plant Food Co.), potassium phosphite analytical standard, and potassium phosphate analytical standard. Each phosphonate formulation was applied at equivalent rates of phosphorous or phosphoric acid, including: 0.06, 0.11, 0.17, 0.22, 0.28 and 0.32 pound/1,000 square feet (0.29, 0.54, 0.83, 1.07, 1.37 and 1.56 grams/square meter).
Treatments in both studies were applied every 14 days from May to August 2010 and from May to September 2011. Applications were made using a hand-held CO2-pressurized sprayer with a single AI9508E flat-fan nozzle calibrated to deliver 2 gallons/1,000 square feet at 40 psi (82 milliliters/square meter at 275 kilopascals). Granular formulations were applied by hand with a shaker jar, and lightly watered in.
Data collection and statistical analyses
Algae infestation was assessed visually as the percent plot area blackened by algae. Data from individual dates were used to calculate the area under the algae development curve (AUADC), which reduces numerous observation dates to a single value representing the overall annual effect of each treatment. Turf quality was also visually assessed in Study 2 during July of each year using a scale of 1 to 9, where 9 represented the best quality and 6 was the minimum acceptable level.
Algae infestation data from Study 1 were analyzed using pre-planned statistical treatment comparisons (that is, contrasts). These statistical comparisons were specifically designed to identify all potential differences between and within the various phosphonate and fungicide treatment groups evaluated. In Study 2, AUADC and turf quality data were analyzed by orthogonal contrasts to identify significant main effects and interactions.
Results
Algae first appeared throughout the study as areas of turf containing black aggregations of filamentous cyanobacteria (Phormidium species) in the lower canopy on June 14, 2010 and Aug. 24, 2011. Infestation of non-treated control plots continued throughout July 2010, with a rapid increase occurring July 14 through 29. Algae increased to its maximum (25% of plot area infested in non-treated plots) by July 29, 2010, and remained at a similar level throughout the remainder of the study in mid-August. In 2011, algae were first observed on Aug. 24 with 19% of plot area infested in non-treated control plots. Thereafter, algae increased to 38% plot area infested in non-treated control plots by Sept. 12.
Study 1: Phosphite sources and conventional fungicides
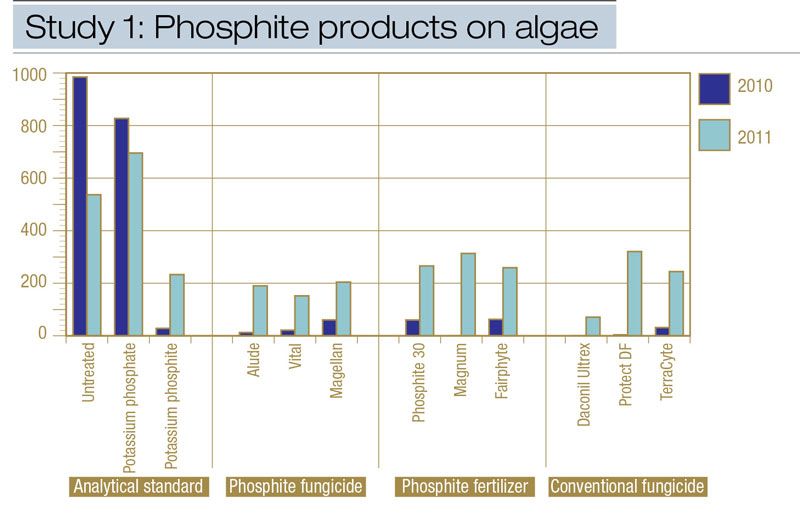
Figure 2. Comparisons from Study 1 of the effects of various phosphite products on algae epidemics, expressed as area under the algae development curve (AUADC), during 2010 and 2011 on creeping bentgrass putting green turf in Storrs, Conn.
All treatments except analytical-grade potassium phosphate reduced algae compared with the non-treated control (Table 2, Figure 2). Analytical-grade potassium phosphite provided equivalent algae suppression compared with commercial phosphite fungicides and phosphite fertilizers during 2010 and 2011. Algae (AUADC) was reduced 52% to 100% and 57% to 97% in plots treated with potassium phosphite compared with potassium phosphate and non-treated control plots throughout the study. Among commercial phosphite products evaluated, fungicides and fertilizers provided similar algae control in 2010 (Table 2, Figure 2). However, phosphite fertilizer-treated plots contained 5% more algae on one observation date (data not shown), increasing the AUADC compared with phosphite fungicides in 2011 (Table 2). Comparisons of various phosphite sources (for example, potassium phosphite, ammonium-potassium phosphite, sodium-ammonium-potassium phosphite), within phosphite fungicides and phosphite fertilizers indicated no significant differences existed among these various phosphite sources in controlling algae throughout the two-year study (Table 2).
Commercial phosphite fungicides and phosphite fertilizers provided algae control equivalent to that provided by conventional algae fungicides throughout the two-year study (Table 2, Figure 2).
Study 2: Phosphonate formulation and rate
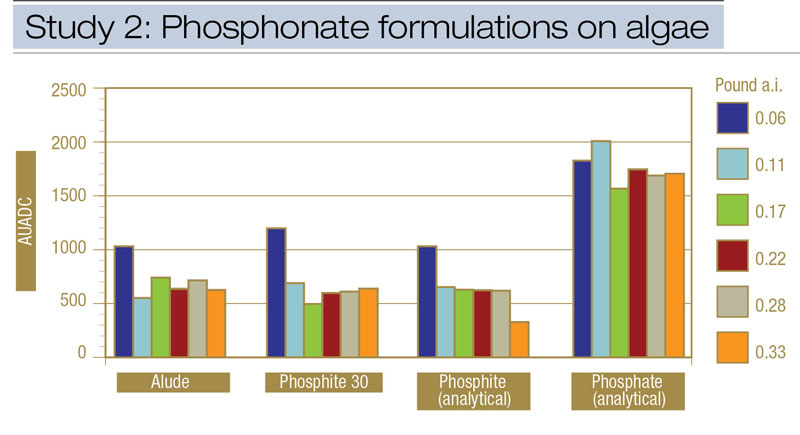
Figure 3. Comparisons from Study 2 of the effects of phosphonate formulations and application rates on algae epidemics, expressed as area under the algae development curve (AUADC), during 2010 and 2011 on creeping bentgrass putting green turf in Storrs, Conn.
Algae severity. Commercially available phosphite fungicide (Alude) and fertilizer (Phosphite 30) formulations were compared with analytical potassium phosphite and potassium phosphate standards at six equivalent rates of the active ingredients phosphorous acid (phosphite) or phosphoric acid (phosphate). Similar to results from Study 1, all potassium phosphite treatments reduced AUADC compared with potassium phosphate (Figure 3). When the amount of active ingredient within Alude, Phosphite 30 and analytical-grade potassium phosphite was applied at the same relative rate, all formulations provided equivalent algae suppression (Figure 4, below). However, a phosphite rate effect did show that phosphite treatments containing ≥ 0.11 pound phosphorous acid/1,000 square feet reduced AUADC compared with 0.06 pound phosphorous/1,000 square feet. Increasing the rate beyond 0.11 pound phosphorous acid/1,000 square feet did not improve control (Figure 3). There were no AUADC differences among any rates of potassium phosphate.

Figure 4. Phosphites can reduce surface algae infestations on creeping bentgrass putting greens.

Figure 5. Increasing the application rate of phosphites can cause tip burn and reduction in turf quality.
Turf quality. Turf quality was reduced in the highest phosphite rates during 2010, but not in 2011. Potassium phosphite (for example, Alude, Phosphite 30, analytical phosphite) applied at ≥ 0.28 pound phosphorous acid/1,000 square feet resulted in slight foliar tip burn and canopy chlorosis during July (Figure 5).
Discussion
In each year of the study, phosphites reduced the cyanobacteria epidemic 57% to 97% on putting green turf compared with non-treated turf. Phosphites applied at 0.11 pound of phosphorous/1,000 square feet were sufficient to reduce cyanobacteria infestations regardless of source or formulation. Higher rates do not improve suppression of algae, and the potential for phytotoxicity to creeping bentgrass increases at rates greater than 0.22 pound phosphorous acid/1,000 square feet.
Phosphite-induced algae suppression was greater during conditions that were moderately favorable for algae in 2010 compared with more conducive conditions in 2011. However, efficacy of conventional fungicides was also variable between these same periods. Interestingly, in all but one rating date during the two-year study (Study 1), commercial phosphite formulations provided control equivalent to the pooled effect of chlorothalonil, mancozeb, and sodium carbonate peroxyhydrate. These results suggest that phosphites may be used as a substitute for conventional fungicides in algae management programs.
Numerous phosphite products derived from various sources (for example, potassium phosphite, sodium-ammonium-potassium phosphite, and ammonium-potassium phosphite) and phosphorous acid are commercially available. This study demonstrated that all phosphite sources evaluated equally reduced cyanobacterial colonization of putting green turf when applied at equivalent rates of phosphorous acid. However, the concentration of phosphorous acid and application rate of commercially formulated phosphite products differ, and can result in varying levels of algae suppression. We observed this in our Study 1, where various phosphite fungicides and fertilizers were applied at the midpoint of their label recommendation. Phosphite fungicides applied in Study 1 generally received 1.7 times more active ingredient per unit area compared with phosphite fertilizers, and resulted in greater algae control during one year of the study (Table 2). Therefore, while all phosphite products have the potential to equally suppress cyanobacteria on putting greens, label-recommended rates for specific products may limit their relative efficacy.
Phosphite competes with phosphate uptake in algae and interferes with normal phosphate-starvation survival responses by preventing utilization of cellular phosphate reserves (5,6,9). Under these conditions, growth can be inhibited because available phosphate is limited, and algae cannot metabolize phosphite. In the current studies, an undetermined species of the cyanobacteria genera Phormidium was found colonizing creeping bentgrass putting green turf. Based on our observations from field studies on the effect of phosphite applications along with previous research on phosphorous metabolism in algal species, it is possible that a similar mechanism is responsible for the phosphite-induced suppression of Phormidium observed in the current studies.
Results from these studies demonstrate that phosphite fungicides and fertilizers can be used to effectively suppress surface colonization of creeping bentgrass putting greens by the cyanobacterium Phormidium species. Control with these materials was comparable to that of conventional fungicides labeled for this purpose. Phosphite formulations and sources applied at equivalent rates of phosphorous acid all provided comparable control of cyanobacteria. However, the potential for phytotoxicity increased when these materials were applied at rates of phosphorous acid ≥ 0.28 pound a.i./square foot. Based on these studies, phosphite products — regardless of source or formulation — applied at 0.11 pound phosphorous acid/1,000 square feet can be used as routine applications to suppress cyanobacteria with minimal risk of phytotoxicity on creeping bentgrass putting greens.
Acknowledgments
This work was partially supported by a GCSAA Cooperative Research Grant from the Environmental Institute for Golf, in affiliation with the Connecticut GCSA, the New Hampshire GCSA, the New England GCSA, the Metropolitan GCSA and the Tri-State Turfgrass Research Foundation.
The research says ...
- Algae, or cyanobacteria, is a common nuisance on greens where low mowing, shade or low fertility reduce turf vigor.
- Phosphite suppression of algae surface infestations on putting green turf was equal to that of conventional fungicides.
- All the commercially available phosphite products tested provided comparable algae control when applied at equivalent rates of active ingredient (that is, phosphorous acid), regardless of source or formulation.
- Excessive phosphite rates (≥ 0.28 pound of phosphorous acid/square foot) can cause slight tip burn.
- Phosphites applied at rates of 0.11 pound of phosphorous acid/1,000 square feet can routinely be used to suppress cyanobacteria with minimal risk of phytotoxicity on creeping bentgrass greens.
Literature cited
- Baldwin, N.A., and B.A. Whitton. 1992. Cyanobacteria and eukaryotic algae in sports turf and amenity grasslands: a review. Journal of Applied Phycology 4:39-47. doi:10.1007/BF00003959
- Cook, P.J., P.J. Landschoot and M.J. Schlossberg. 2009a. Inhibition of Pythium spp. and suppression of Pythium blight of turfgrasses with phosphonate fungicides. Plant Disease 93:809-814. doi:10.1094/PDIS-93-8-0809
- Cook, P.J., P.J. Landschoot and M. Schlossberg. 2009b. Suppression of anthracnose basal rot and improved putting green quality with phosphonate fungicides. International Turfgrass Society Research Journal 11:181-194.
- Dempsey, J.J., I.D. Wilson, P.T.N. Spencer-Phillips and D.L. Arnold. 2012. Suppression of Microdochium nivale by potassium phosphite in cool-season turfgrasses. Acta Agriculturae Scandinavica 62:70-78. doi:10.1080/09064710.2012.681392
- Lee, T.M., P.F. Tsai, Y.T. Shyu and F. Sheu. 2005. The effects of phosphite on phosphate starvation responses of Ulva lactuca (Ulvales, Chlorophyta). Journal of Phycology 41:975-982. doi:10.1111/j.1529-8817.2005.00119.x
- Loera-Quezada M.M., M.A. Leyva-González, D. López-Arredondo and L. Herrera-Estrella. 2015. Phosphite cannot be used as a phosphorus source but is non-toxic for microalgae. Plant Science 231:124-130. doi:10.1016/j.plantsci.2014.11.015
- McDonald, A.E., B.R. Grant and W.C. Plaxton. 2009. Phosphite (phosphorous acid): Its relevance in the environment and agriculture and influence on plant phosphate starvation response. Journal of Plant Nutrition 24:1505-1519. doi:10.1081/PLN-100106017
- Tredway, L.P., L.J. Stowell and W.D. Gelernter. 2006. Yellow spot and the potential role of cyanobacteria as turfgrass pathogens. Golf Course Management 74(11):83-86.
- Zhang, J., J. Geng, H. Ren, J. Luo, A. Zhang and X. Wang. 2011. Physiological and biochemical responses of Microcystis aeruginosa to phosphite. Chemosphere 85:1325-1330. doi:10.1016/j.chemosphere.2011.07.049
John C. Inguagiato is an assistant professor in the Department of Plant Science and Landscape Architecture at the University of Connecticut, Storrs, Conn. John E. Kaminski is an associate professor, and Timothy T. Lulis is a research technologist in the Department of Plant Science at Penn State University, University Park, Pa.