
Five golf courses in Pennsylvania and New York were selected based on their history of imidacloprid applications and reported failures in white grub control in the previous year. Photo by Ben McGraw
White grubs, the larvae of scarab beetles (Coleoptera: Scarabaeidae), are some of the most damaging insect pests of turfgrasses worldwide (3). Populations feeding on roots weaken the turf, leaving it susceptible to drought and other stresses. Additionally, grub infestations often attract predators (e.g. skunks and raccoons), which often cause extensive damage when digging up the turf in search of food. Managing white grub populations has historically been a significant challenge due to their subterranean feeding habits, which makes early detection difficult and can leave turf vulnerable to irreversible damage before infestations are noticed (1).
Imidacloprid, a neonicotinoid insecticide, has been widely used since the mid-1990s for its ability to preventatively control white grubs (4). As a systemic insecticide, imidacloprid is taken up by the roots and translocated throughout the plant, affecting grubs as they feed. Its long residual activity has made it a popular choice for season-long protection with a single application, eliminating the need for frequent monitoring and repeat treatments (2). However, at the time of the study, some turfgrass managers in the northeastern United States reported imidacloprid failures on sites where the insecticide had been used annually. Reports commonly mentioned damage to treated rough-mown areas, whereas adjacent shorter-mown areas (e.g. tees, fairways) were unharmed.
Given the disparity in damage between roughs and adjacent fairways within the site, we explored the potential of thatch accumulation to create a barrier that prevents imidacloprid from reaching the root zone, where grubs feed, and translocating the plant. In short-mown areas, such as golf course fairways, thatch is often managed through regular aerification and topdressing, whereas in higher-mown areas, like roughs, these management practices are not practiced due to the expense and practicality of performing on large areas. We hypothesized that thicker thatch could reduce the efficacy of imidacloprid by limiting its movement into the root zone, leading to higher grub populations in these areas. This hypothesis was examined in both field surveys on golf courses reporting imidacloprid failures and greenhouse experiments designed to measure imidacloprid’s translocation through turf with varying levels of thatch. The objectives of this study are twofold: (1) To analyze the spatial relationship between thatch thickness and white grub populations in the field, and (2) to determine whether thatch impedes the movement of imidacloprid in a controlled greenhouse setting. We hope that by understanding these dynamics, turfgrass managers can make more informed decisions regarding insecticide applications and thatch management practices to optimize white grub control.
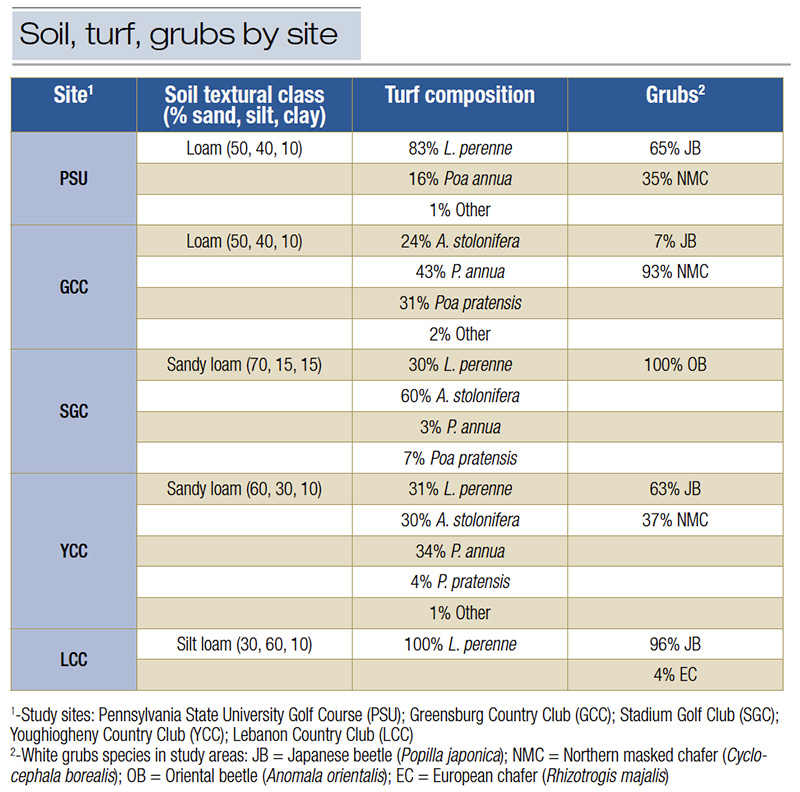
Table 1. Soil texture, turf and white grub species at turfgrass sites reporting imidacloprid failure.
Materials and methods
Field surveys
Site selection
Five golf courses in Pennsylvania and New York were selected based on their history of imidacloprid applications and reported failures in white grub control in the previous year (Table 1). Each site had been applying imidacloprid for over a decade to prevent white grub infestations. The selected sites represented areas with noticeable turf damage in roughs (long-mown) but where adjacent fairways (short-mown) remained largely unaffected.
Spatial analysis and associations
At each golf course, a grid system was established in a turf area that contained both the damaged rough and adjacent fairway areas. The grid was composed of 400 sample plots, each measuring 29.5 inches × 29.5 inches (75 centimeters × 75 centimeters). The grid was equal parts fairway (0.49-0.59 inch/1.25-1.5 centimeters) and rough-mown (1.97-2.76 inches/5-7 centimeters) plots (200 plots from each). A standard golf course cup cutter (3.94 inches diameter × 5.9 inches in depth/10 centimeters × 15 centimeters) was used to collect turf cores from each grid. Each core’s thatch-mat levels were measured using a micrometer. Following measurements, cores were destructively sampled for white grub larvae, and the number and species were recorded.
Spatial Analysis by Distance Indices (SADIE) was employed to analyze the spatial distribution of white grubs, thatch thickness and turfgrass species at each site (Table 2). SADIE is a statistical tool used to detect patterns of aggregation (clustering) or uniformity in spatial data. It works by calculating an index of aggregation (Ia) that quantifies how much the distribution of variable in the dataset (e.g. white grubs, thatch) deviates from randomness. Ia values equal to, greater than and less than 1 indicate a random, aggregated and uniform distribution, respectively. A randomization procedure allows for significance testing on the calculated index’s departure from randomness (Pa). The null hypothesis, that the counts within the sampled area are randomly distributed with respect to each other, may be rejected when Pa < 0.025 (accept the alternative hypothesis for aggregation) or Pa > 0.975 (accept the alternative hypothesis for uniformity). The spatially referenced count data form clusters of either patches (large counts in close proximity to one another) or gaps (zeroes or small counts in close proximity). The program also allows for testing whether two individual datasets with the same x, y coordinates are spatially correlated by generating an index of association (X), along with an associated probability of the index’s departure from randomness (Px). X values significantly greater than zero are considered associated populations (patches corresponding with patches, gaps with gaps); X values significantly less than zero are considered dissociated populations (patches corresponding with gaps); and values not significantly different from zero are considered randomly distributed in respect to one another. X values > 0 are considered significantly associated when p < 0.025 and significantly dissociated with negative X values when p > 0.975. Spatial relatedness (X) between white grub larvae and site characteristics and the associated probability level (Pa) were calculated for each site.
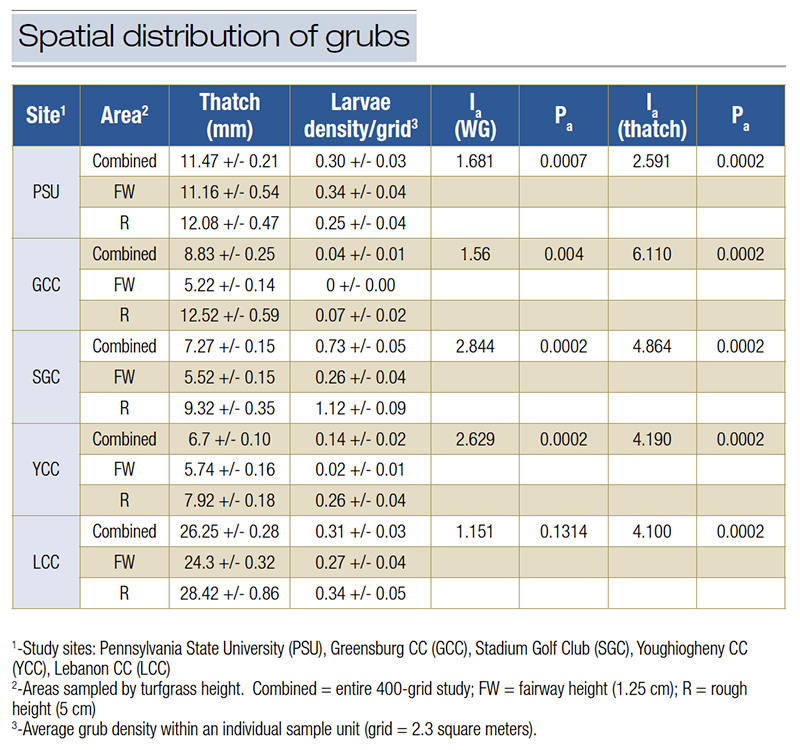
Table 2. Spatial distribution of white grubs in study sites with perceived imidacloprid failure. Ia and Pa values indicate the aggregation value of the overall spatial pattern and the associated significance test of the spatial pattern’s departure from randomness. Ia values indicate an aggregated (> 1), uniform (< 1), and random distribution (close to 1). Significance of the spatial aggregation is assumed at P < 0.025
Greenhouse experiments monitoring imidacloprid movement
Greenhouse trials were conducted to determine whether thatch would impede the movement of imidacloprid into the soil and be translocated through the plant. Perennial ryegrass (Lolium perenne) turf-soil cores were collected from Lebanon Country Club using a golf course cup cutter. Thatch levels were measured, and cores were placed into intermediate (0.2-0.4-inch/5-10-millimeter) and high thatch (> 0.8-inch/20-millimeter) groups. To create a “minimal thatch” condition (< 0.04-inch/1-millimeter), turf was grown on soils from a subset of collected turf cores.
Imidacloprid was applied to each turf plug at the recommended label rate for preventive white grub control (0.00019 ounce active ingredient per square foot/59.2 milligrams active ingredient per square meter) in a carrier volume of 0.05 gallon per acre (0.47 liter per hectare), followed by 0.2 inch (0.5 centimeter) of irrigation. Soil, root and leaf tissue samples were collected from the turf plugs at various time intervals — 7, 14, 28 and 56 days after treatment (DAT). Imidacloprid concentrations were measured using two analytical methods: high-performance liquid chromatography (HPLC) and enzyme-linked immunosorbent assay (ELISA). HPLC is a highly sensitive and accurate technique used to detect and quantify chemical compounds in various substrates. ELISA is a more cost-effective and simpler alternative to HPLC and was assessed as a potential alternative for future studies where cost or time constraints may limit the use of more sophisticated techniques.
Statistical analysis
Imidacloprid concentrations in plant tissues and soil were log-transformed to normalize the variance before statistical analysis. One-way analysis of variance (ANOVA) was used to compare the mean imidacloprid concentrations across the different thatch treatments. Tukey’s honest significant difference (HSD) test was applied to separate means when significant differences were detected. Additionally, two-way ANOVA was used to evaluate interactions between thatch thickness and imidacloprid concentrations in plant tissues and soil.
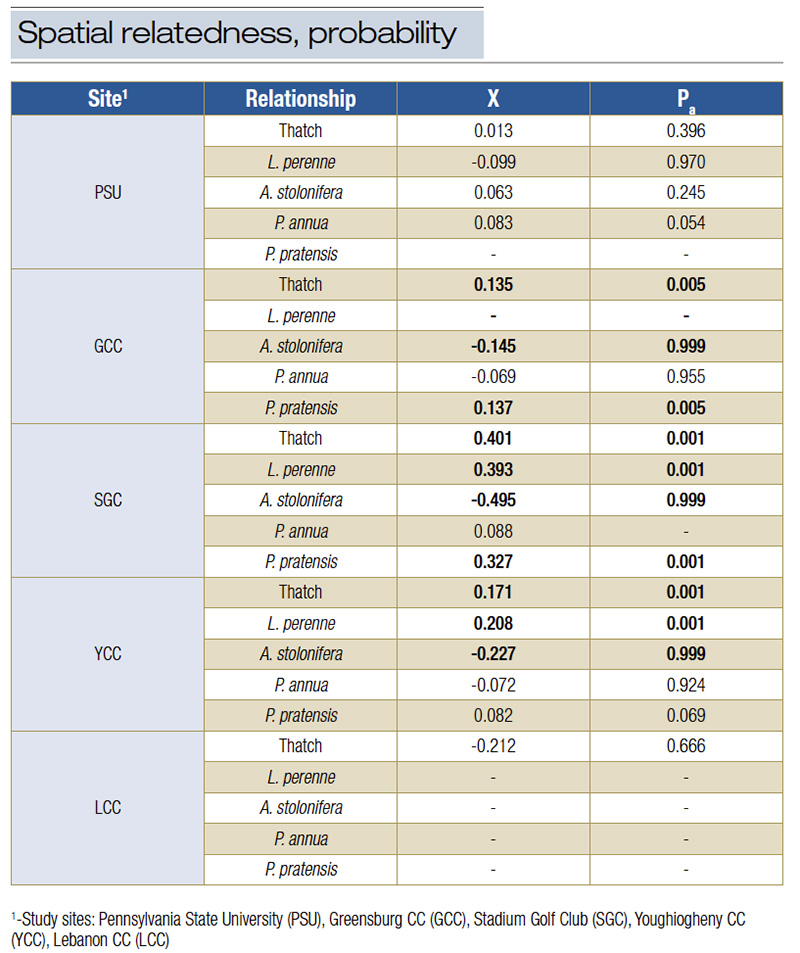
Table 3. Spatial relatedness (X) between white grub larvae and site characteristics and the associated probability level (Pa). X values greater than 0 are considered significantly associated when P < 0.025 and significantly dissociated with negative X values and when P > 0.975. Bolded figures represent significant spatial patterns.
Results
Field surveys
White grub densities across entire sites were far below commonly accepted thresholds (≤ 1.68 grubs per square meter), although isolated areas within each study site exceeded established thresholds (110-636 grubs per square meter). In four of the five golf courses, significantly more white grubs were found in rough-mown areas compared to fairways despite both areas being treated with imidacloprid. Grub densities in roughs ranged from 0.12 to 2.06 grubs per square meter, while fairways exhibited significantly lower densities, ranging from 0 to 0.6 grubs per square meter. Thatch thickness measurements showed a distinct difference between rough and fairway areas across all sites, except for one golf course (Greensburg Country Club), where no significant difference was observed. Average thatch thickness in rough-mown turf ranged from 0.26 to 1 inch (6.7 to 26.25 millimeters). In contrast, fairways had significantly lower thatch, averaging between 0.21 and 0.49 inch (5.22 and 12.52 millimeters).
Spatial analysis and associations
White grub spatial distribution was significantly aggregated (Ia ≥ 1.56; p ≤ 0.004) at four sites. Thatch thickness was found to be significantly aggregated at all study sites (Ia ≥ 2.6; Pa ≤ 0.0013). Strong associations were found between the spatial patterns of white grubs and L. perenne and Poa pratensis L. at three of four sites, while a significant disassociation (X ≤ −0.145; p ≥ 0.999) was found between white grub larval density and creeping bentgrass (Agrostis stolonifera L.) at all three sites where the species was present.
White grub distribution patterns (Table 3) were significantly associated with thatch thickness at three of five study sites (X ≥ 0.1351; p ≤ 0.01). A Pearson’s correlation test of combined data revealed no correlation or level at which thatch thickness yielded higher white grub densities (X = 0.002; p = 0.98). When analyzed individually, only one of the five courses (SGC) had a significant relationship between increasing white grub densities with increases in thatch thickness (X = 0.47; p = 0.001).
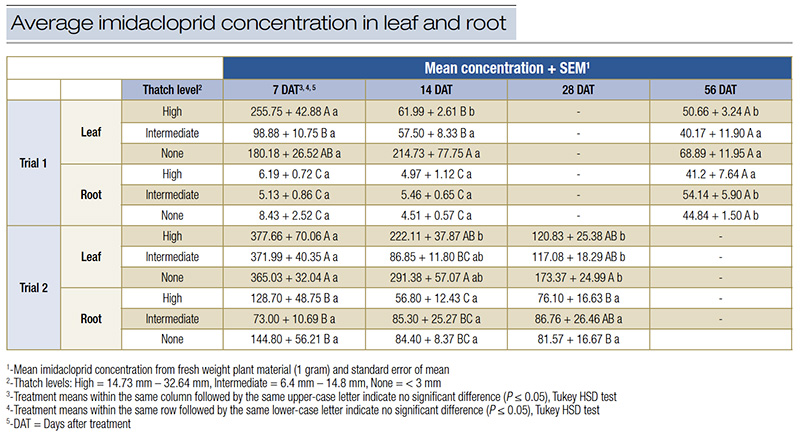
Table 4. Average imidacloprid concentration (+/- SEM) in treated perennial ryegrass leaf and root tissue as estimated by HPLC (Trial 1) or ELISA (Trial 2).
Greenhouse experiments monitoring imidacloprid movement
Estimates of imidacloprid concentrations in turfgrass plants and soil obtained through ELISA were comparable to those determined by the more precise and established HPLC method. While HPLC demonstrated greater sensitivity at lower concentrations, ELISA provided similar trends in concentration estimates, suggesting it may be a useful approach for certain applications. This consistency between methods highlights the potential of ELISA as a practical alternative, particularly in contexts where resource constraints limit the feasibility of using HPLC.
Imidacloprid concentrations were significantly higher in leaf tissues compared to root tissues across all thatch treatments. This pattern was consistent across all sampling intervals. At 7 DAT, imidacloprid concentrations in leaf tissues (Table 4) ranged from 98.88 to 255.75 parts per billion (ppb), while root tissue concentrations were significantly lower, ranging from 5.13 to 8.43 ppb. By 56 days after treatment, imidacloprid concentrations had decreased in both leaves and roots, with leaf concentrations ranging from 40.17 to 68.89 ppb and root concentrations ranging from 41.2 to 54.14 ppb. Neither HPLC nor ELISA detected significant differences in imidacloprid concentrations attributable to thatch thickness. This finding was consistent across all time points, suggesting that thicker thatch does not impede the translocation of imidacloprid in the plant.
Imidacloprid concentrations in the soil (Table 5) were generally lower than in plant tissues, with no clear pattern indicating a significant effect of thatch thickness. At 7 days after treatment, soil imidacloprid concentrations ranged from 1.36 to 7.20 ppb, and by 56 days, concentrations were between 17.9 and 24.3 ppb.
Conclusions
SADIE revealed that white grubs exhibited a highly aggregated distribution at four out of five study sites, with concentrations particularly notable in rough-mown areas. The analysis demonstrated a strong association between white grub densities and thicker thatch layers. While thicker thatch was associated with higher grub densities, this relationship does not appear to result from thatch impeding imidacloprid movement into the soil. Imidacloprid concentration analyses, performed using both ELISA and HPLC, indicated that thatch treatments did not significantly affect imidacloprid levels in plant tissues or soil. Despite minor statistical differences across treatments or over time, neither method revealed a consistent pattern linking thatch thickness to imidacloprid movement or accumulation. Instead, environmental factors, such as moisture retention in thicker thatch, may create more favorable conditions for grub survival and beetle oviposition. These findings emphasize the complexity of interactions within the turfgrass ecosystem and the importance of considering both chemical and environmental factors in grub management strategies.
For turfgrass managers, these findings underscore the importance of managing thatch as part of an integrated pest management strategy. While thick thatch may create favorable conditions for grubs, it does not appear to block imidacloprid from reaching the root zone. However, maintaining optimal thatch levels through aerification and topdressing can help reduce pest habitats and improve overall turf health.
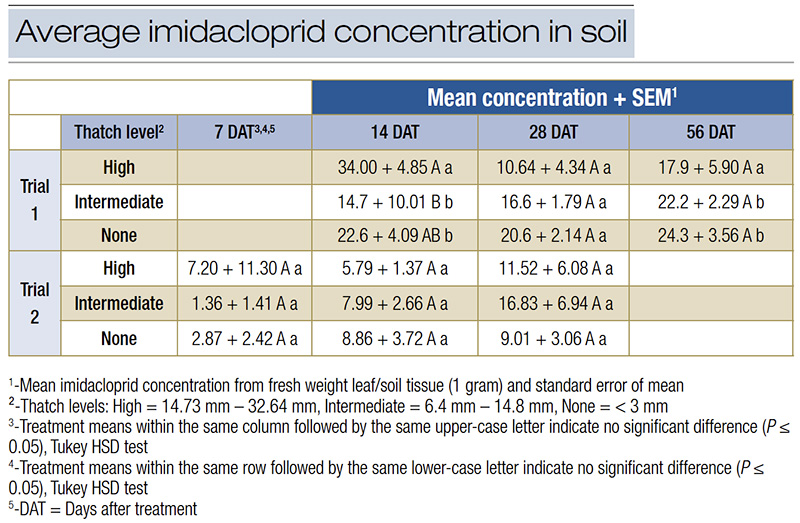
Table 5. Average imidacloprid concentration (+/- SEM) in treated soil measure with High Performance Liquid Chromatography (HPLC) or ELISA (Trial 2).
The research says
Literature cited
- Potter, D.A. 1998. Destructive turfgrass insects: Biology, diagnosis, and control. Ann Arbor Press.
- Simon-Delso, N., V. Amaral-Rogers, L.P. Belzunces, J.M. Bonmatin, M. Chagnon, C. Downs, et al. 2015. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environmental Science and Pollution Research, 22(1):5-34 (https://doi.org/10.1007/s11356-014-3470-y).
- Vittum, P.J. 2020. Turfgrass insects of the United States and Canada. Cornell University Press.
- Zenger, J.T., and T.J. Gibb. 2000. Impact of four insecticides on Japanese beetle (Coleoptera: Scarabaeidae) egg predators and white grubs in turfgrass. Journal of Economic Entomology, 94(1):145-149 (https://doi.org/10.1603/0022-0493-94.1.145).
Benjamin A. McGraw (bam53@psu.edu) is an associate professor of turfgrass science in the Department of Plant Science at Pennsylvania State University, University Park, and Andrew Huling is at USDA APHIS Plant Protection and Quarantine, Raleigh, N.C.