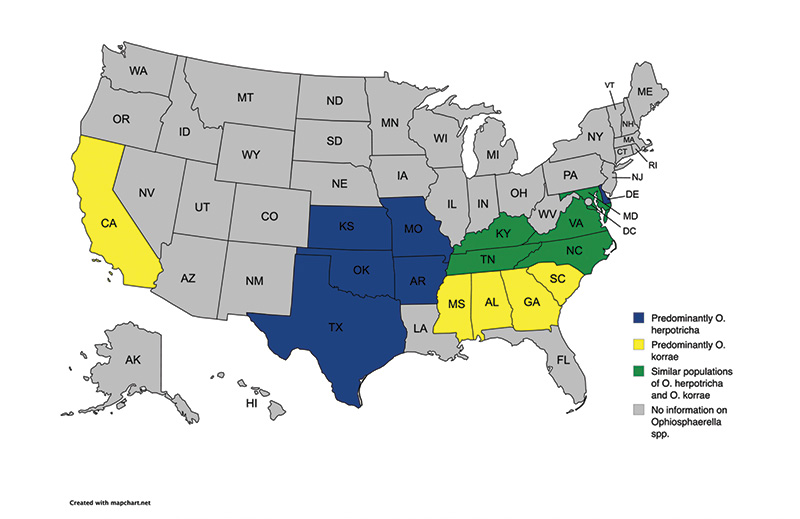
Figure 1. Current understanding of Ophiosphaerella species distribution throughout the United States. *Ophiosphaerella narmari has been isolated in California, Kansas, North Carolina, Oklahoma and Texas. Figure adapted from Hutchens et al. (2024a) paper.
Spring dead spot (SDS), caused by Ophiosphaerella spp., is one of the most challenging diseases to manage for golf course superintendents growing bermudagrass in the transition zone. Symptoms appear during spring green-up as circular, necrotic and sunken patches that often take weeks, or even months, to recover. The Ophiosphaerella spp. that cause the disease infect the crowns, rhizomes, stolons and roots of turfgrass plants, particularly bermudagrass. The pathogen can infect bermudagrass throughout most of the year, but the infection during the fall as bermudagrass approaches dormancy predisposes the plant to winter injury (17). The fall infection in conjunction with freezing winter temperatures exacerbates SDS symptoms in the spring. This poses a challenge for superintendents managing SDS because symptoms appear months after infection.
The challenges of SDS have prompted extensive research over the years. All research efforts until 2009 were summed up thoroughly in a Tredway et al. (2009) article. However, since 2009, a surge of SDS research efforts have been undertaken, leading to nearly 20 peer-reviewed publications in the last 15 years (Table 1). This research has focused primarily on 1) characterizing the host range of the Ophiosphaerella spp. that cause SDS, 2) a deeper understanding of the biology of the Ophiosphaerella spp. that cause SDS, 3) distribution of SDS and epidemiological factors that influence where SDS occurs, and 4) chemical and cultural management strategies to prevent and recover from SDS. This article will flesh out the recent discoveries related to SDS and hopefully provide some useful information for readers to better understand SDS and how to manage it.
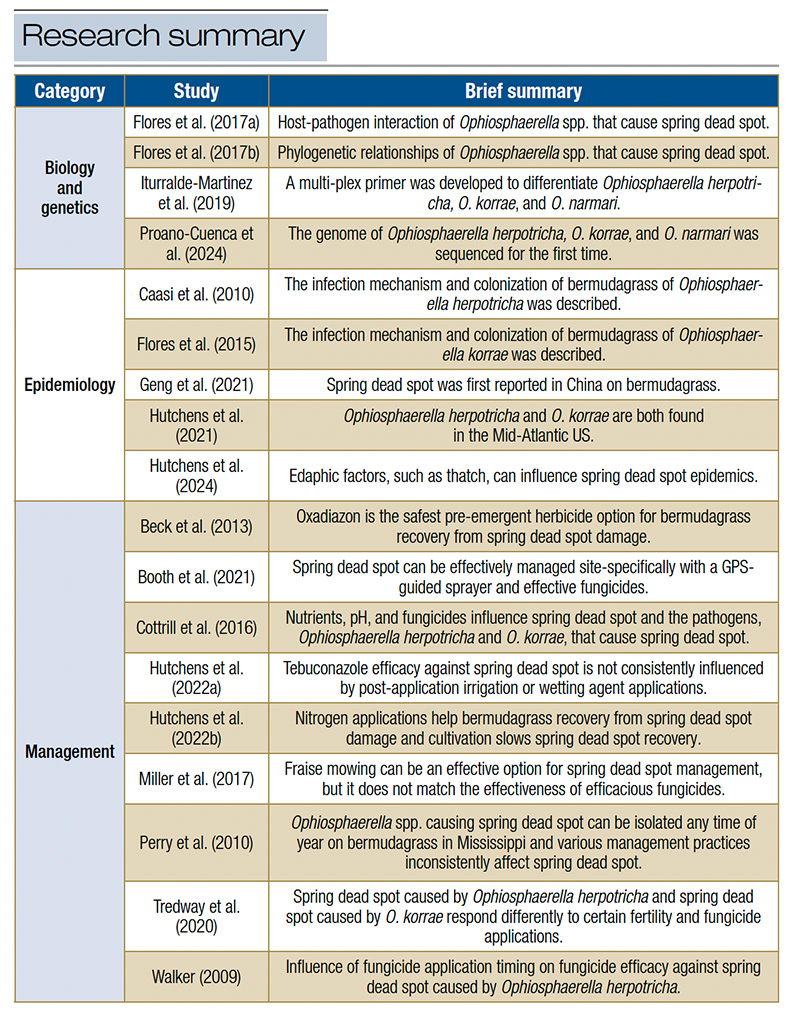
Table 1. Summary of spring dead spot research papers published since Tredway et al. (2009) spring dead spot review paper. Table adapted from Hutchens et al. (2024a) paper.
Host range and biology of the Ophiosphaerella spp. that cause spring dead spot
Spring dead spot is a fairly unusual disease in that it is caused by more than one fungal species. There are three primary pathogens that cause SDS: O. herpotricha, O. korrae and O. narmari. Spring dead spot can be caused by any one of these species, individually, or as a complex of multiple Ophiosphaerella spp. The two most common Ophiosphaerella spp. that golf course superintendents in North America face are O. herpotricha and O. korrae, whereas O. narmari has primarily been an issue in Australia and New Zealand (24). Although these three species are closely related, they often respond differently to management practices — this will be further addressed in the “Management strategies for spring dead spot” section of this article.
Bermudagrass is the host plant for O. herpotricha, O. korrae and O. narmari that gets the most attention, probably because it is the most widely grown turfgrass species on golf courses in the U.S. and because it is particularly susceptible to SDS (19, 24). However, O. herpotricha, O. korrae and O. narmari account for a much wider host range (Table 2). Bermudagrass, buffalograss, kikuyugrass, St. Augustinegrass and zoysiagrass are all warm-season turfgrasses that are susceptible to one or multiple Ophiosphaerella spp. that cause SDS (3, 5, 6, 8, 20, 21, 22, 25). Recent discoveries by Flores et al. (2017) have advanced our understanding of the host range of the Ophiosphaerella spp. that cause SDS.
Beyond host range, there are other key factors about the biology of the SDS pathogens that have been characterized in the last 15 years. The genomes of O. herpotricha, O. korrae and O. narmari have recently been sequenced (18), and new multiplex primers for rapid identification have been constructed (15). These new developments will aid in more rapid and accurate identification of SDS and the Ophiosphaerella spp. that cause it, which will lead to more informed and effective management decisions for golf course superintendents.
The infection mechanism of O. herpotricha and O. korrae has also been described over the last 15 years (3, 5). Ophiosphaerella herpotricha and O. korrae infect bermudagrass root and stolon tissue through direct hyphal penetration, and the pathogen likely survives over the summer in the stolon tissue. Although the infection mechanism is now understood, damage to bermudagrass via the host-pathogen interaction may not be as straightforward as previously thought. This process is being studied by researchers at Oklahoma State University to fully understand the host-pathogen interaction between Ophiosphaerella spp. and bermudagrass (6). More to come on that in the future.
Where is spring dead spot found?
This question can be asked in two different and more specific ways. 1) Where is SDS found across the U.S. and globally? 2) Where do SDS outbreaks appear on a golf course — i.e., are there driving factors in the soil and environment that lead to SDS epidemics in specific locations on a golf course?
Where is SDS found across the U.S. and globally?
Spring dead spot has been documented across many parts of the globe, particularly where bermudagrass is grown and winter dormancy occurs. The disease has been found in Australia, Europe, New Zealand, North America and South America. Only recently was the disease documented and characterized in Asia, specifically China (7). Within the U.S., the distribution of O. herpotricha, O. korrae and O. narmari varies. In California and throughout much of the southeastern U.S., O. korrae is the predominant species that causes SDS (17, 24) (Figure 1). In the Midwest, however, O. herpotricha has been the predominant species, historically (4). The geographic distribution of Ophiosphaerella spp. was only recently described for the Mid-Atlantic U.S., and researchers determined that both O. herpotricha and O. korrae appear throughout the region (11). Interestingly, the location of the parent material for the bermudagrass cultivar planted at each site throughout the Mid-Atlantic region was often an indicator of whether O. herpotricha or O. korrae was predominant at that site. A golf course or athletic field that has a bermudagrass cultivar whose parent material is from the Midwest is more likely to have O. herpotricha, while a golf course or athletic field that has a bermudagrass cultivar whose parent material is from the Southeast is more likely to have O. korrae (21). It is also important to note that O. narmari is not commonly found in the U.S.
Where do SDS outbreaks appear on a golf course?
The factors that drive SDS epidemics were not thoroughly described until recently. When we think about disease development, we must refer to the disease triangle. The disease triangle is a representation of the necessary components for a disease to develop in a plant — a turfgrass system, in our case. The three components of the disease triangle are pathogen, host and conducive environment. The conducive environment component includes temperature, humidity, soil moisture, organic matter content, thatch, pH, nutrient content, etc. But which of these factors drive SDS epidemics? A recent Hutchens et al. (2024c) study determined that many factors are correlated with SDS epidemics, and one factor that stood out was thatch depth. Areas with a deeper thatch depth (i.e., more thatch) tended to have more SDS than areas with shallower thatch depths (i.e., less thatch). This provides valuable information on how to manage SDS. Management practices that reduce thatch should, in turn, reduce SDS pressure. Another interesting finding from the study was that SDS epidemics appear in clusters, or an aggregated pattern, from year to year. Understanding where SDS outbreaks occur and why they occur in those particular places can help golf course superintendents make site-specific, prescriptive management decisions.
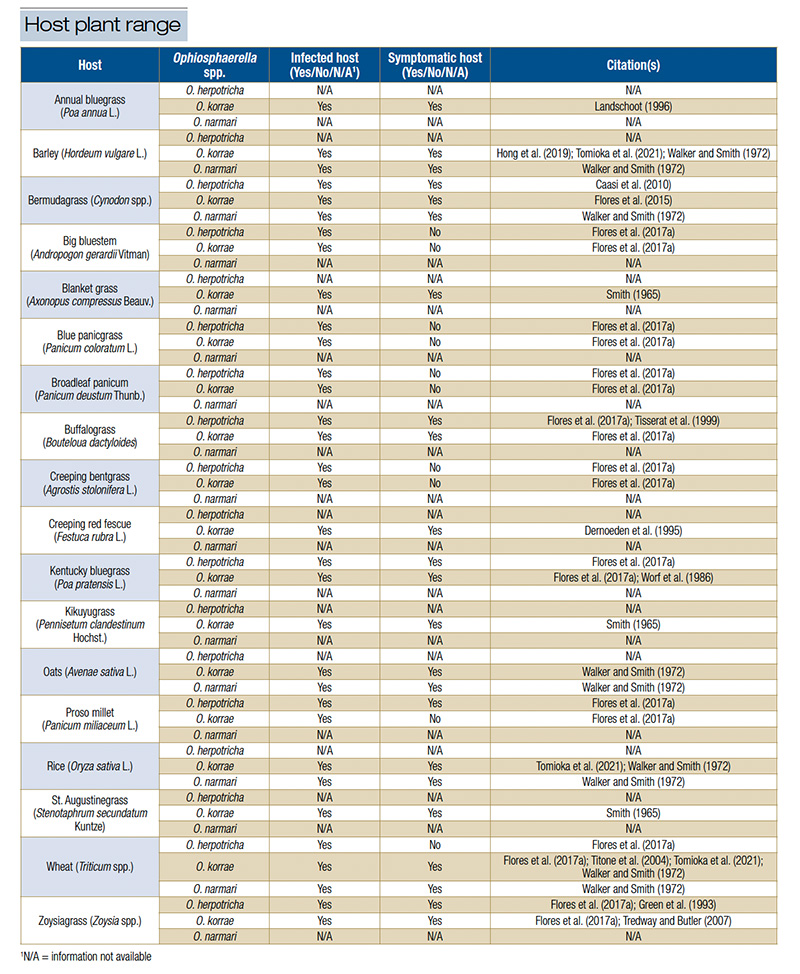
Table 2. Host plant range for Ophiosphaerella herpotricha, O. korrae and O. narmari. Table adapted from Hutchens et al. (2024a) paper.
Management strategies for spring dead spot
Managing SDS should be an integrated approach that includes both cultural and chemical practices. Myriad cultural and chemical practices have been studied for their effects on SDS, but, in many cases, results have been inconsistent. Inconsistent effects of management practices for soilborne diseases in turfgrass such as SDS are not uncommon, but SDS is fairly unusual in that multiple pathogen species can cause the disease, adding a layer of complexity to management approaches. Although O. herpotricha and O. korrae belong to the same genus and both cause SDS, they often respond differently to many cultural and chemical practices, so that must be taken into consideration when managing SDS.
Cultural practices for managing SDS
Spring dead spot, as mentioned earlier, is positively correlated to thatch depth, so reducing thatch is recommended. Management practices that are known for reducing thatch, such as fraise mowing, can also reduce SDS if performed preventatively (16). However, aggressive cultivation is not recommended for recovering bermudagrass from SDS damage. Instead, quick-release nitrogen applications in the absence of cultivation is actually recommended to help bermudagrass more rapidly recover from SDS (10). Applying preemergent herbicides that are shoot-absorbed, like oxadiazon, instead of root-absorbed is also an effective tactic for more rapidly recovering bermudagrass from SDS damage (1).
Fertilizer source also plays a role in preventing SDS, and it can be species-specific in its effects on SDS (23). Calcium nitrate has been demonstrated to inhibit SDS caused by O. korrae, and ammonium sulfate can inhibit SDS caused by O. herpotricha (23). These results are not always consistent between studies, though (4). Other nutrients can affect SDS as well, but not consistently. For example, the effects of sulfur and manganese on SDS have both produced mixed results in various studies, warranting further investigation of these two nutrients (4, 17). The best current recommendation for SDS management with nutrients is to maintain a balanced soil nutrition by avoiding nutrient deficiencies and excessive nutrients.
Chemical practices for managing SDS
Fungicidal suppression of SDS has improved drastically over the last decade. Best management practices for fungicide selection, application timing and application method have all been studied in detail in recent years. However, similar to the effects of certain cultural practices on O. herpotricha and O. korrae, fungicides can differentially suppress the two species (23). Although this presents a challenge, the research community still has made major strides into more efficiently and effectively managing SDS with fungicides.
Proper fungicide selection is crucial for optimal suppression of any turfgrass disease, including SDS. The succinate dehydrogenase-inhibiting (SDHI) and demethylation-inhibiting (DMI) fungicides are generally most effective against SDS (2, 9, 16, 26). However, these fungicides do not suppress SDS unless they are applied at the correct time. The most recent data shows fungicides for SDS should be applied in the fall when 0-to-4-inch soil temperatures range from 55-65 F (13-18 C) (13). Beyond proper application timing, fungicides should be irrigated in after application, even though post-application irrigation does not always enhance fungicide efficacy against SDS (9).
Where do we go from here?
Research on SDS has come a long way in the last 15 years or so. This includes studies on the biology and epidemiology of the disease, as well as preventative and recuperative management practices to mitigate SDS damage. However, more research is necessary to improve our understanding of SDS and formulate more efficient management strategies for the disease. Future research efforts should focus on leveraging advanced technologies, such as remote and ground-based sensors, GPS-guided sprayers and spreaders, and advanced statistical models to site-specifically and prescriptively treat SDS. Future research efforts should also attempt to provide greater understanding of the biology of the Ophiosphaerella species that cause SDS.
The research says
- Understanding where SDS outbreaks occur and why they occur in those particular places can help golf course superintendents make site-specific, prescriptive management decisions.
- The best current recommendation for SDS management with nutrients is to maintain a balanced soil nutrition by avoiding nutrient deficiencies and excessive nutrients.
- Future research efforts should focus on leveraging advanced technologies such as remote and ground-based sensors, GPS-guided sprayers and spreaders, and advanced statistical models to site-specifically and prescriptively treat SDS.
Literature cited
- Beck, L.L., T. Cooper, A.J. Hephner, C.M. Straw and G.M. Henry. 2013. Effect of preemergence herbicides on the recovery of bermudagrass from spring dead spot. Applied Turfgrass Science 10(1):1-7 (https://doi.org/10.1094/ATS-2013-0328-01-RS).
- Booth, J.C., D. Sullivan, S.A. Askew, K. Kochersberger and D.S. McCall. 2021. Investigating targeted spring dead spot management via aerial mapping and precision-guided fungicide applications. Crop Science 61(5):3134-3144 (https://doi.org/10.1002/csc2.20623).
- Caasi, O.C., N.R. Walker, S.M. Marek, J.N. Enis and T.K. Mitchell. 2010. Infection and colonization of turf-type bermudagrass by Ophiosphaerella herpotricha expressing green or red fluorescent proteins. Phytopathology 100(5):415-423. https://doi.org/10.1094/PHYTO-100-5-0415
- Cottrill, D.J., D.T. Earlywine and G.L. Miller. 2016. Assessment of nitrogen source, sulfur, and fall fungicide applications on the management of spring dead spot of bermudagrass. Plant Disease 100(2):473-482 (https://doi.org/10.1094/PDIS-05-15-0565-RE).
- Flores, F.J., S.M. Marek, J.A. Anderson, T.K. Mitchell and N.R. Walker. 2015. Infection and colonization of several bermudagrasses by Ophiosphaerella korrae. Phytopathology 105(5):656-661 (https://doi.org/10.1094/PHYTO-07-14-0205-R).
- Flores, F.J., S.M. Marek, J.A. Anderson, T.K. Mitchell, M. Moreno-Zambrano and N.R. Walker. 2017a. Reactive oxygen species production and alternative hosts of spring dead spot-causing fungi. International Turfgrass Society Research Journal 13(1):213-224 (https://doi.org/10.2134/itsrj2016.06.0480).
- Geng, J.M., S. Jiang and J. Hu. 2021. First report of Ophiosphaerella narmari causing spring dead spot of hybrid bermudagrass in China. Plant Disease 105(12):4153 (https://doi.org/10.1094/PDIS-03-21-0535-PDN).
- Green, D.E., J.D. Fry, J.C. Pair and N.A. Tisserat. 1993. Pathogenicity of Rhizoctonia solani AG-2-2 and Ophiosphaerella herpotricha on zoysiagrass. Plant Disease 77:1040-1044.
- Hutchens, W.J., J.C. Booth, J.R. Doherty, J.A. Roberts and D.S. McCall. 2022a. Influence of post-application irrigation and soil surfactants on tebuconazole efficacy against spring dead spot. Crop Protection 156:105961 (https://doi.org/10.1016/j.cropro.2022.105961).
- Hutchens, W.J., J.C. Booth, J.M. Goatley and D.S. McCall. 2022b. Cultivation and fertility practices influence hybrid bermudagrass recovery from spring dead spot damage. HortScience 57(2):332-336 (https://doi.org/10.21273/HORTSCI16235-21).
- Hutchens, W.J., C.A. Henderson, E.A. Bush, J.P. Kerns and D.S. McCall. 2021. Geographic distribution of Ophiosphaerella species in the Mid-Atlantic United States. Plant Health Progress 23(1):93-100 (https://doi.org/10.1094/PHP-04-21-0076-S).
- Hutchens, W.J., J.K. Anders, E.L. Butler, J.P. Kerns, D.S. McCall, G.L. Miller and N.R. Walker. 2024a. Fifteen years of findings: Advancements in spring dead spot research from 2009 to 2024. Crop Science 1-12 (https://doi.org/10.1002/csc2.21367).
- Hutchens, W.J., J.C. Booth, J.M. Goatley, T.L. Roberson and D.S. McCall. 2024b. Optimizing fungicide application timing for spring dead spot based on soil temperature and season. Crop Science 1-13 (https://doi.org/10.1002/csc2.21411).
- Hutchens, W.J., C. Henderson, C.M. Straw, J.M. Goatley, J.P. Kerns, M. Nita, D. Sullivan and D.S. McCall. 2024c. Environmental and edaphic factors that influence spring dead spot epidemics. Phytopathology 114(1):155-163 (https://doi.org/10.1094/PHYTO-10-22-0398-R).
- Iturralde Martinez, J.F., F.J. Flores, A.R. Koch, C.D. Garzon and N.R. Walker. 2019. Multiplex end-point PCR for the detection of three species of Ophiosphaerella causing spring dead spot of bermudagrass. Plant Disease 103(8):2010-2014 (https://doi.org/10.1094/PDIS-10-18-1727-RE).
- Miller, G.L., D.T. Earlywine and B.S. Fresenburg. 2017. Effect of fraze mowing on spring dead spot caused by Ophiosphaerella herpotricha of bermudagrass. International Turfgrass Society Research Journal 13(1):225-228 (https://doi.org/10.2134/itsrj2016.10.0839).
- Perry, D.H., M. Tomaso-Peterson and R. Baird. 2010. Seasonal variation in frequency of isolation of Ophiosphaerella korrae from bermudagrass roots in Mississippi and pathogenicity and optimal growth of selected isolates. Mycopathologia 169:395-402 (https://doi.org/10.1007/s11046-010-9273-x).
- Proano-Cuenca, F., N. Graf-Grachet, C. Garzon, Y. Wu, S. Marek and N. Walker. 2024. Draft genome sequences of 11 isolates of Ophiosphaerella spp., the causal agents of several turfgrass diseases including spring dead spot of bermudagrass and necrotic ring spot of Kentucky bluegrass. PhytoFrontiers 4(2) (https://doi.org/10.1094/PHYTOFR-02-23-0019-A).
- Shaddox, T.W., J.B. Unruh, M.E. Johnson, C.D. Brown and G. Stacey. 2023. Turfgrass use on U.S. golf courses. HortTechnology 33(4): 367-376 (https://doi.org/10.21273/HORTTECH05238-23).
- Smith, A.M. 1965. Ophiobolus herpotrichus: A cause of spring dead spot in couch turf. Agricultural Gazette of New South Wales 76:753-758.
- Tisserat, N., H. Wetzel, J. Fry and D.L. Martin. 1999. Spring dead spot of buffalograss caused by Ophiosphaerella herpotricha in Kansas and Oklahoma. Plant Disease 83(2):199 (https://doi.org/10.1094/PDIS.1999.83.2.199D).
- Tredway, L.P., and E.L. Butler. 2007. First report of spring dead spot of zoysiagrass caused by Ophiosphaerella korrae in the United States. Plant Disease 91(12):1684 (https://doi.org/10.1094/PDIS-91-12-1684A).
- Tredway, L.P., M.D. Soika, E.L. Butler and J.P. Kerns. 2020. Impact of nitrogen source, fall fertilizers, and preventative fungicides on spring dead spot caused by Ophiosphaerella korrae and O. herpotricha. Crop Science 61(5):3187-3196 (https://doi.org/10.1002/csc2.20306).
- Tredway, L.P., M. Tomaso-Peterson, H. Perry and N.R. Walker. 2009. Spring dead spot of bermudagrass: A challenge for researchers and turfgrass managers. Plant Health Progress 10(1) (https://doi.org/10.1094/PHP-2009-0710-01-RV).
- Walker, J., and A.M. Smith. 1972. Leptosphaeria narmari and L. korrae spp. nov., two long-spored pathogens of grasses in Australia. Transactions of the British Mycological Society 58(3):459-466 (https://doi.org/10.1016/S0007-1536(72)80094-9).
- Walker, N.R. 2009. Influence of fungicide application timings on the management of bermudagrass spring dead spot caused by Ophiosphaerella herpotricha. Plant Disease 93(12):1341-1345 (https://doi.org/10.1094/PDIS-93-12-1341).
Wendell Hutchens (wendellh@uark.edu) is an assistant professor of turfgrass science at the University of Arkansas, Fayetteville.