
Figure 1. Chlorosis and thinning of a Tifway 419 hybrid bermudagrass (Cynodon dactylon [L.] Pers. x Cynodon transvaalensis Burtt-Davy) fairway in response to soil with pH less than 4.0 at the 2-inch (5-centimeter) depth in west central Florida. Replacement of injured 419 with fresh sod (dark strips) was done to improve turfgrass quality, but ultimately the new sod also failed. Multiple treatments with dolomitic limestone at 1 ton per acre (2.24 tons per hectare) corrected the problem and improved turfgrass quality. Photos by William Berndt
Acidity is an environmental pressure that helps determine the fitness of soil for turfgrasses. The level of acidity in a soil is estimated by determining soil reaction or pH (3). Soils having a pH of less than 7.0 are acidic, and soils having a pH greater
than 7.0 are basic or alkaline; soil is considered neutral when pH = 7.0 at 77 F (25 C) and a pressure of 1 bar (Table 1). Most golf course superintendents know this, but what superintendents may not fully appreciate are the problems associated with
acidic soils. Excessive acidity may cause a decline in turfgrass quality (Figure 1) by stressing or damaging root systems (Figure 2) due to the presence of toxic ions and reduced availability of nutrients (6, 17). The objective of this article is
to review how soils become acidic, describe the problems associated with acid soils, and describe the best management practices (BMPs) for treating acid soils — those soils having low values of pH or high levels of acidity.
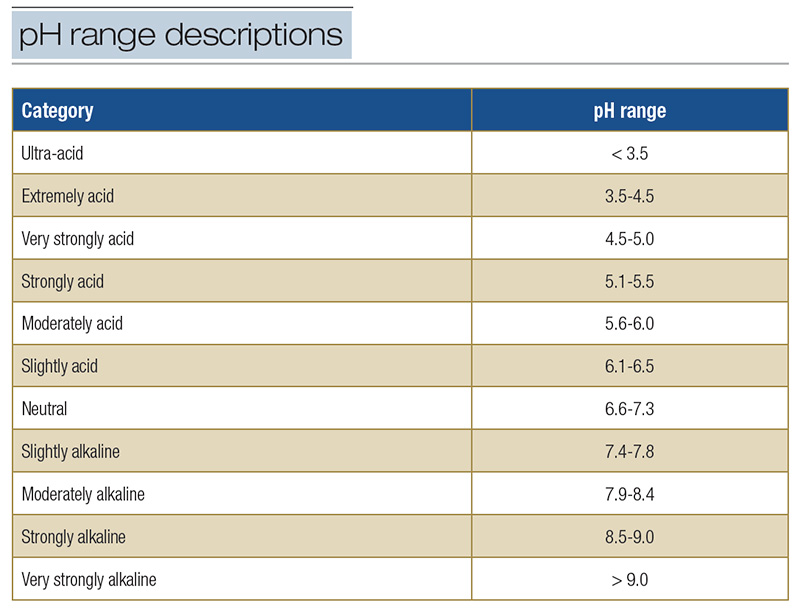
Table 1. Descriptive terms used to designate pH ranges in soil.
How do soils become acidic?
Acidification of soil can occur naturally and by the performance of certain cultural practices. Carbon dioxide (CO2) in the atmosphere and CO2 released during soil respiration (i.e., from decay of soil organic matter) is a natural source of soil acidity.
The CO2 is soluble in water and diffuses into soil solution where it reacts with water to become carbonic acid (H2CO3). The carbonic acid then dissociates, forming bicarbonate ion (HCO3-) and releasing hydrogen ions (H+) to soil solution:
CO2 + H2O ↔ H2CO3 ↔ HCO3- + H+
Hydrogen ions (H+) are acidity; measured soil pH is the activity (concentration) of H+ in soil solution. High levels of CO2 in soil equate to higher levels of carbonic acid in soil solution, meaning more H+, hence increased potential for a lower value
of soil pH.
Rainwater is another natural source of acidity. The pH of rainwater is 5.65 (12) because water vapor in the atmosphere is in equilibrium with atmospheric CO2; rainwater naturally contains carbonic acid. Rainwater may have an even lower pH (i.e., 3.5 to
5.0) due to emission of byproducts of fossil fuel combustion (i.e., sulfur dioxide and nitrogen oxide). Rainwater is also in equilibrium with any fossil fuel byproduct gases in the atmosphere. Rainfall having pH less than about 5.0 is termed acid
rain.

Figure 2. Visible root necrosis of a goosegrass (Eleusine indica [L.] Gaertn.) plant growing in a Tifway 419 hybrid bermudagrass fairway (Figure 1) in response to soil with pH < 4.0 at the 2-inch (5-centimeter) depth in westcentral Florida.
Irrigation water may be naturally acidic. Irrigation water from ground sources in central South Carolina had pH = 2-3 (William Berndt, personal observation). This groundwater was so acidic it caused aluminum overhead irrigation infrastructure on a sod
field to corrode and fail and ultimately led to cementation of the sod field soil, which compromised growth of the sod. Leaching soil with excess water, whether by rainfall or irrigation events, can lower the pH of a soil when the volume of water
is large and acidic, liming does not occur, and supplemental fertility does not replace basic nutrients subject to leaching (i.e., K+, Mg2+, Ca2+). The H+ in acidic water dislodges and replaces basic nutrients adsorbed to cation exchange sites, leaving
soil devoid of nutrients. Excessive rainfall is one reason tropical soils can be acidic and infertile.
Plants are another natural source of acidity. Plant roots excrete acids, including oxalic acid (H2C2O4) and other organic acids as part of the rhizosphere effect. Oxalic acid discharged to soil solution from plant roots dissociates, releasing H+ and potentially
lowering pH:
H2C2O4 ↔ HC2O4- + H+
Plants may also discharge H+ directly through ion channels during nutrient uptake. Pumping H+ out of plant roots is central to the physical process of generating energy for importing nutrients from soil solution into root cells.
Common cultural practices may also lead to acidification of soil. The application of acidifying fertilizers containing ammonium (NH4+) and acidifying soil amendments such as elemental sulfur (S0) may contribute directly to soil acidity via oxidation (reaction
with oxygen) of ammonium and sulfur, respectively:
2 NH4+ + 4 O2 → 2 NO3- + 4 H+ + 2 H2O
2 S0 + 3 O2 → 2 H2SO4 → HSO4- + H+ → SO42- + H+
In both reactions, H+ is released. Both reactions also consume soil oxygen potentially generating an anaerobic soil environment. Commonly used nutrient carriers are acidifying (Table 2); calcium nitrate and potassium nitrate tend to raise soil pH.
Early turfgrass literature (1920-1940) advocated using acidifying fertilizers to improve turfgrass growth. This was termed acid theory, a belief of the time that bentgrasses (Agrostis spp.) needed decidedly acid soils (18). Early newsletters suggested
acid soil was desirable for growth of turfgrasses (32) and that soil can never be too acid for growing bentgrass, which was said to do well at pH 3.7 (33). Acidifying soil was encouraged for better turf growth with fewer weeds. Applying ammonium sulfate
alone or with other fertilizers reduced soil pH, improved bentgrass quality and mitigated growth of crabgrass (Digitaria spp.) (36). Ammonium sulfate applied at 10 pounds per 1,000 square feet (488 kilograms per hectare) or more was recommended for
acidifying soil during construction of putting greens before seeding (bentgrass) or planting bentgrass stolons (34). Repeated applications of acidifying fertilizer “almost miraculously” improved poor, weed-infested turf (18).
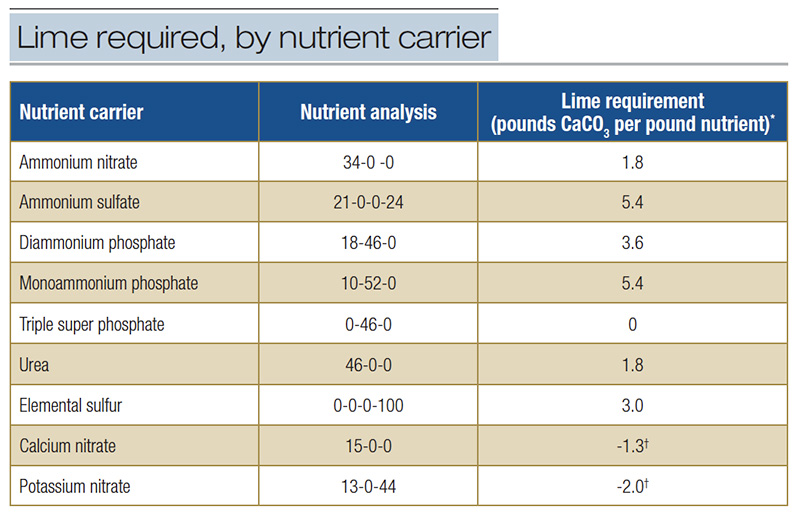
Table 2. Lime required to neutralize the acidity associated with nutrient carriers (22). Choice of nutrient carrier can influence pH.
*Pounds pure calcium carbonate (CaCO3) required to neutralize acidity from one pound of applied nutrient. †Excess alkalinity from applying one pound of nutrient
Renouncing acid theory
Pushback on acid theory occurred due to toxicity associated with free aluminum (Al3+) and deficiency of Ca2+ and Mg2+ in acidic soil (8). Noer (20) stated the “acid era” in turf maintenance on golf courses died with the grass when soil became
too acid during hot, humid weather. Severity of dollar spot (Clarireedia spp.) and brown patch (Rhizoctonia spp.) was reduced by liming bentgrass turf growing on very acid soil (35); liming increases soil pH. Low soil pH interfered with the ability
of grasses to utilize ammonia, causing it to accumulate and become toxic (19). Acid soil was also linked to poor root development and accrual of thatch (5, 14, 19, 23, 27, 30) (Figure 3). Arnon and Johnson (2) reported complete failure of root growth
of common bermudagrass (Cynodon spp.) in solution culture at pH = 3. Thatch accrual may be favored by acid soil, possibly due to antagonism of decay-inducing microbes (6). Callahan et al. (5) reported thatch accumulation was double on soil with
pH less than 4.0 compared to soil with pH greater than 5.0.
More recently, applying ammonium sulfate lowered soil pH from 5.45 to 4.98 over two years, resulting in an increase in severity of anthracnose in annual bluegrass (Poa annua L. f. reptans [Hausskn.] T. Koyama), possibly due to toxic effects of Al3+ (28).
Annual bluegrass grown in greens mix with pH = 5.5 exhibited morphological differences compared to plants grown in two other soils with pH 6.5 and 8.1 (11). Plants were shorter, took longer to produce a first panicle, had fewer panicles and fewer
branches on each panicle, and produced fewer seeds that were lighter in weight. Observed effects may have been due to decreased availability of P, K+, Mg2+ and Ca2+ compared to soils with higher pH, finer texture and greater water-holding capacity

Figure 3. Accrual of thatch-mat taken from a TifEagle hybrid bermudagrass putting green in southwest Florida. Reports exist in the literature linking thatch accumulation to soil acidity.
Free aluminum is toxic
One consequence of acidic soil is the release of toxic free Al3+ to soil solution (31). This occurs because acidic solutions with pH less than about 5.5 cause dissolution of aluminum minerals such as gibbsite (γ-Al(OH)3). Free Al3+ in soil solution
is toxic to plants because it a powerful oxidizer, meaning that due to its electronic nature, it strips substances like root tissues of electrons, causing tissues to deteriorate. Exposure to Al3+ inhibits cell division, cell elongation and enzyme
processes in roots, decreases root respiration, and interferes with uptake and transport of Ca2+, Mg2+ and water (31). Free Al3+ alters root cell morphology and damages root apices (1). Cell walls may be the site of maximum Al3+ accumulation (i.e.,
cells take Al3+ up), where it compromises cell wall stability (1). Root-absorbed Al3+ leads to production of reactive oxygen species (ROS), including superoxide anion (O2-), which physically damages biomolecules like lipids and nucleic acids (7, 25).
Free Al3+ alters cell membrane potential and impacts the activities of nutrient transporters in root tissues (15). These kinds of effects can be induced within minutes at micromolar concentrations of Al3+ (24).
In addition to being toxic, the chemistry of Al3+ is such that it reacts with water in soil solution forming what is called a solvation complex (Figure 4). This means that each free Al3+ ion forms bonds with six water molecules to produce a hydrated aluminum
ion (i.e., Al(H2O)63+). Hydrated aluminum is an acidic trivalent cation that perpetuates additional acidity when soil pH is low. The hydrated aluminum ion generates additional acidity via hydrolysis reactions (i.e., reactions with water) that liberate
H+ from each of the adsorbed water molecules (31):
Al(H2O)63+ + H2O ↔ Al(OH)(H2O)52+ + H+
Al(OH)(H2O)52+ + H2O ↔ Al(OH)2(H2O)4+ + H+
Al(OH)2(H2O)4+ + H2O ↔ Al(OH)3(H2O)3 + H+
Al(OH)3(H2O)3 + H2O ↔ Al(OH)4(H2O)2- + H+
In these reactions, H+ is released in successive steps as pH rises unless there is a source of neutralizing hydroxyl ions (i.e., OH-). This is part of the reason aluminum has been called the seat of soil acidity (13). Al3+ is released from minerals due
to low pH, and its chemistry helps maintain low pH, leading to dissolution of more minerals containing aluminum, iron and manganese. Like Al3+, cations like iron (Fe3+) and manganese (Mn2+) are also acidic and can become toxic in low-pH environments.
Collectively, these three acidic cations are termed Lewis acids. Carbon dioxide and H+ are also Lewis acids.
Free Al3+ may also impact cation exchange capacity (i.e., CEC). At pH less than 5.5, free Al3+ begins to saturate cation exchange sites. At pH = 4, nearly 100% of exchange sites may be saturated with Al3+ (9). Trivalent Al3+ along with monovalent H+ displace
nutrient cations and are strongly adsorbed by the exchange sites at low pH. When the CEC is saturated with Al3+ and H+, there is reduced availability of nutrient cations including Ca2+, Mg2+, K+ and NH4+ available for plant growth. After being displaced,
these cations leach away with drainage water. In addition, the chemistry of acid soils may impact the nature of variable charge sites, creating positive (+) charge sites and effectively reducing CEC. In other words, pH-dependent CEC is negatively
affected. This can be important, as the CEC for thatch and soil humus may be 90%-100% pH-dependent (6).
Aluminum may also impact microbial communities because it is toxic to microbes. When Pseudomonas flourescens, which are bacteria common to turfgrass soils, are exposed to Al3+, the aerobic formation of ATP is disrupted, impacting the Kreb’s
cycle, which is essential for aerobic respiration (16). Al3+ is an inhibitor of the Kreb’s cycle (10).
Aluminum toxicity in cyanobacterium (Nostoc linckia) increased as pH decreased from 7.5 to 4.5; respiration was completely stopped at pH 6 and pH 4.5 when coupled with Al3+ at 0.8 and 0.6 millimolar, respectively (22).
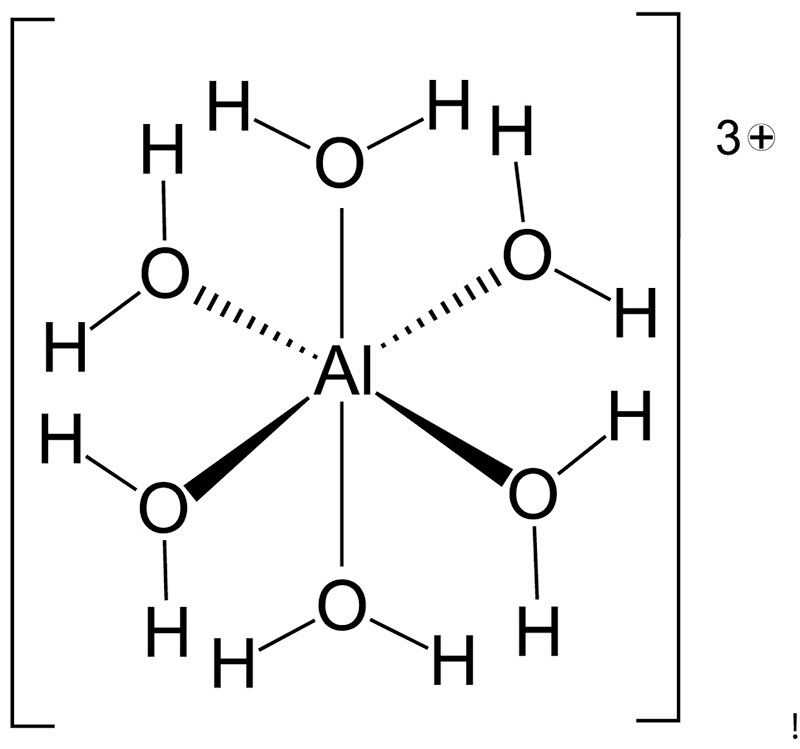
Figure 4. At low soil pH free Al3+ released from dissolution of aluminum minerals forms bonds with six water molecules water creating a solvation complex called hexaquaaluminum or Al(H2O)63+. This solvation complex is based on bonding of six water molecules to Al3+. In addition to being toxic this molecule is highly acidic, releasing H+ from each adsorbed H2O in stepwise fashion further lowering the pH of soil. Other acidic solvation complexes common to turfgrass soils include those complexes based on iron III (Fe3+), iron II (Fe2+) and manganese II (Mn2+).
Lime mitigates acid soil toxicity
Applying lime (i.e., calcium carbonate, CaCO3) neutralizes soil acidity, increases pH and helps alleviate toxicity of Al3+ and other Lewis acids (Figure 5) associated with acidic soils.
This was demonstrated early on. Liming was reported to overcome the toxic effects of Al3+ and accrual of residues of sulfate (SO42-) following use of ammonium sulfate as fertilizer (26). Liming soil to mitigate impacts of low pH has been advocated
over the years (6, 21, 29, 37). Liming is a BMP for acid soils.
The neutralization reaction of lime is based on the reaction of calcium carbonate (or other liming materials) with water, producing hydroxyl anion (OH-):
CaCO3 + H2O → Ca2+ + HCO3− + OH−
Neutralization occurs when OH− reacts with H+ to form water; the OH- binds the acidity:
H+ + OH- → H2O
Calcium does not participate directly in neutralization reactions, but its presence may help to displace H+ and Al3+ adsorbed to cation exchange sites. Liming materials available to mitigate aluminum toxicity have differing neutralizing values (Table
3). The speed with which neutralization reactions occur with these materials varies with neutralizing value, material fineness and material solubility.
Finer, more soluble materials neutralize acidity at a faster rate (Table 4). Coarse liming material has less surface area to react with soil moisture dissolving at a slower rate, hence a slower effect on neutralizing acidity.
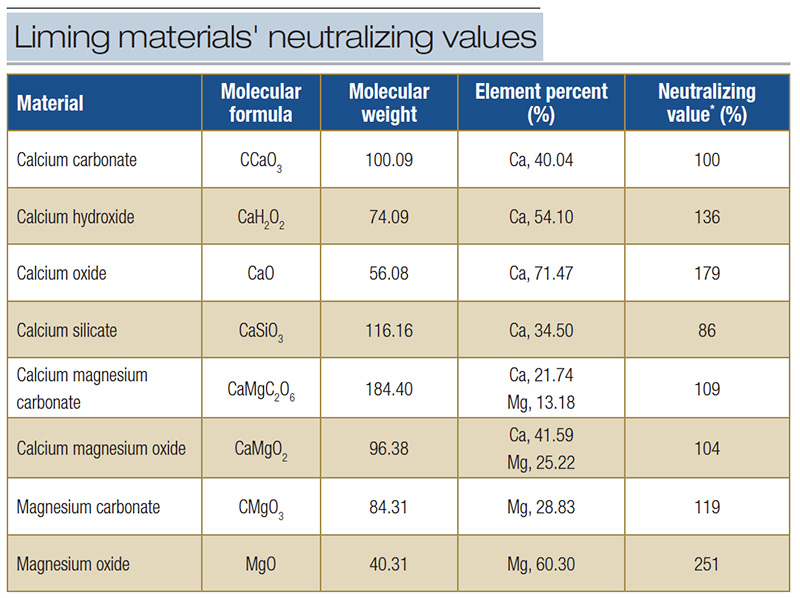
Table 3. Neutralizing values of common liming materials as calcium carbonate equivalent (CCE). *Calcium carbonate equivalent (CCE).
Lime requirement
The lime requirement is the amount of liming material needed to change the acidity of a specified soil to a specified level (4, 9, 31). Soil testing laboratories typically determine lime requirement by determining the ability of a soil to resist changes
in pH (i.e., its buffering ability). From that information, a rate of lime is recommended by the laboratory. Typical rates of lime may be 0.5 to 1 ton per acre (1,235.5 to 2,471.1 kilograms per hectare).
Soil laboratories make lime recommendations based on addition of a soil sample to a pH buffer solution and determining the change in pH in response to the soil. Buffers are materials that resist changes in pH. This is known as the buffer pH method (31).
The more the soil lowers the pH of the buffer-soil suspension (i.e., the more acidic the soil is), the greater the lime requirement. Popular buffers used for this are the Shoemaker-McLean-Pratt (SMP) buffer, the Adams-Evans buffer and the Mehlich
buffer, and various modifications of these buffer solutions.
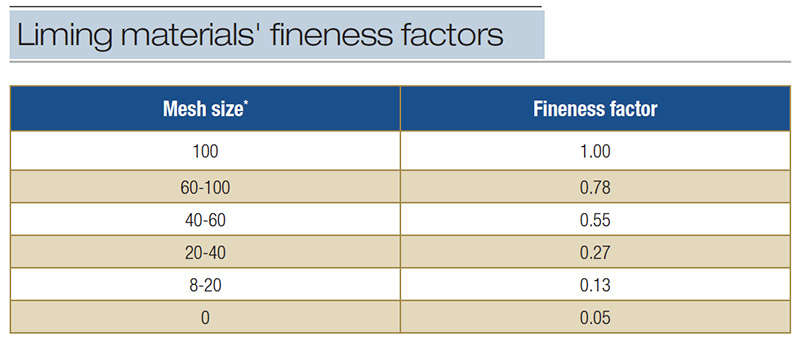
Table 4. Fineness factors associated with particle size of liming materials used to give effective calcium carbonate equivalent (CCE) (from A&L Canada Laboratories Inc., 2013). *Material passing mesh size
Best management practices
The starting point for golf course superintendents is to have a reputable laboratory establish both soil pH and buffer pH for the soil of interest. When having pH measurements done by a lab, make sure to ask what the extractant is and what the soil-to-extractant
ratio is (3) so that over time one can compare apples to apples and not apples to oranges. If the pH is slightly acidic (6-7), there is probably no need to do anything. The pH buffer test will confirm this. However, if pH is lower (5.5 and lower),
there may be a need to apply lime. Keep in mind that 30% to 50% of the world’s arable land has pH less than 5.5 (1). If liming is needed, the lab will specify the rate to use in conjunction with the pH buffer test.
If soil pH is low and liming is recommended, it may be prudent to assess the levels of aluminum in soil and in plant tissues. At pH near 5.5 and lower, plants may absorb Al3+, causing stress and predisposing turfgrass plants to stress-related diseases,
such as anthracnose, and stress-related disorders, such as thinning and chlorosis. If soil pH is low and liming is recommended, it also may be prudent to implement other BMPs to mitigate the effects of acidity:
- Water-in liming materials immediately after application to get the materials into the soil. Liming materials are calcium or magnesium salts, so avoiding extended leaf contact is desirable, especially when temperatures and humidity are high.
- Reduce the use of acidifying fertilizers and soil amendments. Ammonium-based fertilizers, elemental sulfur, iron-based sulfates and acid injection products like urea sulfate are acidifying. Calcium nitrate may be a good alternative.
- Evaluate the pH of irrigation water. Acidic sources of water not only serve to acidify soil but also strip soil of basic nutrients, resulting in infertility. Evaluate irrigation water for aluminum content.
- Examine the base saturation percentages of H+ and aluminum. These acidic cations have no nutritional value for turfgrasses. Also, have plant tissue (clippings) analyzed for aluminum content.
- Maintain adequate levels of available soil phosphorus. At low pH, aluminum and iron may precipitate (bond) with phosphorus, rendering it unavailable to plants and microbes.
- Manage salinity effectively. Introducing salts into acidic soils, whether by irrigation water or fertilizers, may serve to displace Al3+ or other acidic cations like Fe3+ adsorbed to cation exchange sites, effectively increasing the acidity of the
soil solution and the potential for negative effects of Lewis acid cations.
- When sampling a soil for pH, it might be prudent to independently sample the top 2 inches, the soil at 3-4 inches, and soil below 4 inches. Sectioning the samples in this way may give better insight into the true pH and a more complete picture of
the root zone.

Figure 5. Application of lime on an annual bluegrass (Poa annua L.) putting green in Dublin, Ireland. Using pelletized, greens-grade forms of lime may reduce drift associated with limestone application.
Conclusions
Management problems with acid soils involve the presence of toxic cations like Al3+ and diminished presence of basic nutrients, including K+, Mg2+ and Ca2+. It’s not the low pH per se that is problematic, although H+ can be directly toxic at very
low pH, and trying to grow plants in an acid bath can be challenging, especially when soil temperatures are high. Rather, the effects of low pH on soil constituents leading to release of toxins and related impacts to CEC and microbial communities
are typically the main problems.
When treating acid soils with lime, the goal should not simply be to raise the pH, because increasing soil pH may not be an easy task. Soils may be highly buffered, meaning that the pH of the soil may be stable, poised at a particular pH for a variety
of reasons. Thus, the effect of liming on pH may only be temporary; after liming, the soil may quickly return to the original pH. Instead, the goal should be to introduce enough OH- into the soil frequently enough to neutralize a portion of the acidity
and thereby help to mitigate the effects of Al3+ and H+ and/or other acidic cations. Sand-based soils like putting greens may have less buffering capacity, meaning the pH may fluctuate more in response to the management it receives. So be aware of
the effects your management plan may have. Potential for acidification in poorly buffered soils is enhanced by fertilizing with ammonium, applying sulfur and or turning on the acid injection system. Liming, in conjunction with other BMPs, helps mitigate
plant stresses imposed by acid soils.
The research says
Literature cited
- Aggarwal, A., B. Ezaki, A. Munjal and B.N. Tripathi. 2015. Physiology and biochemistry of aluminum toxicity and tolerance in crops. In Stress Responses in Plants. B.N. Tripathi and M. Muller (eds.). (https://doi.org/10.1007/978-3-319-13368-3_2).
- Arnon, D.I., and C.M. Johnson. 1942. Influence of hydrogen ion concentration on the growth of higher plants under controlled conditions. Plant Physiology 17(4):525-539 (https://doi.org/10.1104/pp.17.4.525).
- Berndt, W.L. 2022. Laboratory measurement of soil pH: What golf course superintendents need to know. Golf Course Management 90(2): 70-73.
- California Fertilizer Association. 1998. Using amendments to correct soil and growing-media problems. Western Fertilizer Handbook 2nd Horticultural Edition. Interstate Publishers, Inc., Danville, Ill.
- Callahan, L.M., W.L. Sanders, J.M. Parham, C.A. Harper, L.D. Lester and E.R. McDonald. 1998. Cultural and chemical controls of thatch and their influence on rootzone nutrients in a bentgrass green. Crop Science 38(1):181-187 (https://doi.org/10.2135/cropsci1998.0011183X003800010030x).
- Carrow, R.N., D.V. Waddington and P.E. Rieke. 2001. Turfgrass soil Fertility and Chemical Problems: Assessment and Management. Page 400. Ann Arbor Press, Chelsea, Mich.
- Chauhan, D.K., V. Yadav, M.Vaculik, W. Grassman, S. Pike, et al. 2021. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Critical Reviews in Biotechnology 41(5):715-730 (https://doi.org/10.1080/07388551.2021.1874282).
- Cox, H.R. 1929. Whether or not to lime — soil acidity tests and their practical uses. Golfdom: The Business Journal of Golf 3(6):21-23.
- Foth, H.D., and B.G. Ellis. 1997. Soil Fertility, Second Ed. Lewis Publishers, Boca Raton, Fla.
- Gasmi, A., M. Peana, M. Arshad, M. Butnariu, A. Menzel and G. Bjørklund. 2021. Krebs cycle: Activators, inhibitors and their role in the modulation of carcinogensis. Archives of Toxicology 95:1161-1178 (https://doi.org/10.1007/s00204-021-02974-9).
- Guertal, E.A. and J.S. McElroy. 2018. Soil type and phosphorus fertilization affect Poa annua growth and seedhead production. Agronomy Journal 110(6):2165-2170 (https://doi.org/10.2134/agronj2018.02.0139).
- Harter, R.D. 2002. Acid soils of the tropics. ECHO Technical Note. ECHO Inc., North Fort Myers, Fla. Online. Available at: http://courses.umass.edu/psoil370/Syllabus-files/Acid_Soils_of_the_Tropics.pdf.
- Jenny, H. 1961. Reflections on the soil acidity merry-go-round. Soil Science Society of America Journal 25(6):428-432 (https://doi.org/10.2136/sssaj1961.03615995002500060006x).
- Johnson, A. 1951. Use of lime. The Bull Sheet 5(6):3.
- Kar, D., A.A. Pradhan and S. Datta. 2021. The role of solute transporters in aluminum toxicity and tolerance. Physiologia Plantarum 171(4):638-652 (https://doi.org/10.1111/ppl.13214).
- Lemire, J., R. Mailloux, C. Auger, D. Whalen and V.D. Appanna. 2010. Pseudomonas fluorescens orchestrates a fine metabolic-balancing act to counter aluminum toxicity. Environmental Microbiology 12(6):1384-1390 (https://doi.org/10.1111/j.1462-2920.2010.02200.x).
- McCarty, L.B., I.R. Rodriguez, B.T. Bunnell and F.C. Waltz. 2003. Fundamentals of Turfgrass and Agricultural Chemistry. Page 375. John Wiley and Sons Inc., Hoboken, N.J.
- Monteith, J. 1932. Soil acidity and lime for bent turf. Bulletin of Green Section of the United States Golf Association 12(5):190-195.
- Musser, H.B. 1962. Turf management. McGraw-Hill Book Company Inc. New York.
- Noer, O.J. 1946. The effect of acidity on turf and the chemistry of acid soils. Golfdom 20(5):26.
- Noer, O.J. 1962. Lime in the life of a plant. The Bull Sheet 15(11):3-5.
- Piña, R.G., and C. Cervantes. 1996. Microbial interactions with aluminum. BioMetals 9(3):311-316 (https://doi.org/10.1007/BF00817932).
- Potter, D.A., B.L. Bridges and F.C. Gordon. 1985. Effect of N fertilization on earthworm and microarthropod populations in Kentucky bluegrass turf. Agronomy Journal 77(3):33-36 (https://doi.org/10.2134/agronj1985.00021962007700030004x).
- Rahman, M.A., S. Lee, H.C. Ji, A.H. Kabir, C.S. Jones and K. Lee. 2018. Importance of mineral nutrition for mitigating aluminum toxicity in plants on acidic soils: Current status and opportunities. International Journal of Molecular Sciences 19(10):3073-3101
(https://doi.org/10.3390/ijms19103073).
- Ranjan, A., R. Sinha, T.R. Sharma, A. Pattanayak and A.K. Singh. 2021. Alleviating aluminum toxicity in plants: implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiologia Plantarum 173(4): 1765-1784
(https://doi.org/10.1111/ppl.13382).
- Reid, M.E. 1932. The effects of soil reaction upon the growth of several types of bent grasses. The Bulletin of the United States Golf Association Green Section 12(5):196-212.
- Sartain, J.B. 1985. Effect of acidity and N source on the growth and thatch accumulation of TifGreen bermudagrass and on soil nutrient retention. Agronomy Journal 77(1):33-36 (https://doi.org/10.2134/agronj1985.00021962007700010009x).
- Schmid, C.J., B.B. Clarke and J.A. Murphy. 2017. Anthracnose severity and annual bluegrass quality as influenced by nitrogen source. Crop Science 57(S1):S-285-S-292 (https://doi.org/10.2135/cropsci2016.06.0494).
- Schmidt, R.E. and J.E. Shoulders. 1971. Fertilization practices and quality turf. USGA Green Section Record 9(6):12-13.
- Smiley, R.W., and M.M. Craven. 1978. Fungicides in Kentucky bluegrass turf: Effects on thatch and pH. Agronomy Journal 70(6):1013-1019 (https://doi.org/10.2134/agronj1978.00021962007000060030x).
- Tisdale, S.L., W.L Nelson and J.D. Beaton. 1985. Soil Fertility and Fertilizers, Fourth Edition. Page 754. Macmillan Publishing Company, N.Y.
- USGA Green Section. 1925a. Sour soil and acid soil. Bulletin of Green Section of the United States Golf Association 5(5):116.
- USGA Green Section. 1925b. Possible superacidity of soil for bentgrass. Bulletin of Green Section of the United States Golf Association 5(12):287.
- USGA Green Section. 1926. Acidifying soil before planting. Bulletin of Green Section of the United States Golf Association 6(9):207.
- USGA Green Section. 1941. Soil acidity. Timely Turf Topics. April:2-3.
- Westover, H.L. 1925. Effects of certain fertilizers on soil acidity, quality of turf, and weed control. Bulletin of the Green Section of the United States Golf Association 5(12):269-271.
- White, C. 1962. Lime, one of the answers. The Golf Course Expert 1(3):2.
William L. Berndt (Leeberndt@aol.com, @Dr_Lee_Berndt) is a consulting agronomist specializing in golf course turfgrass management. He has a doctorate in botany and plant pathology from Michigan State University and is the author of numerous turfgrass
publications. A former college professor, he actively consults on golf courses, conducts and publishes turfgrass research and performs expert witness work.