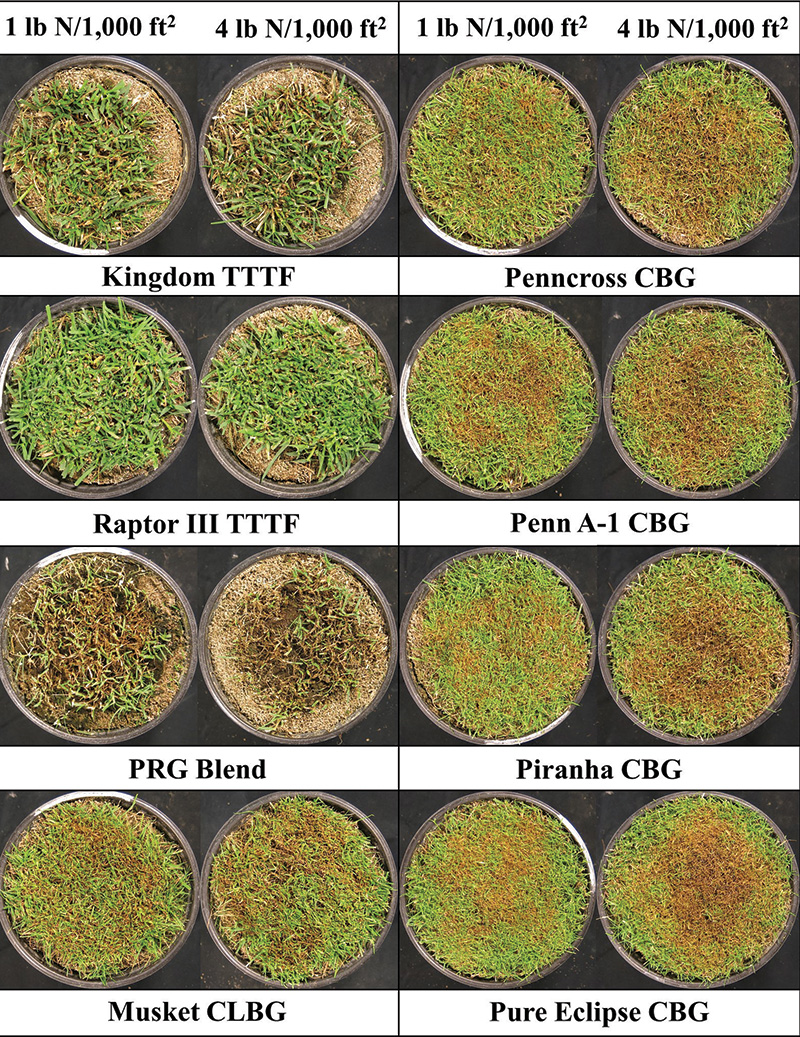
Figure 1. Example of four cool-season species — creeping bentgrass (CBG), colonial bentgrass (CLBG), perennial ryegrass (PRG) and turf-type tall fescue (TTTF) — and cultivars 26 days after Rhizoctonia solani inoculation in a high-humidity, enclosed chamber located inside a controlled environment facility in West Lafayette, Ind., during Experiment 2 when fertilized with a total of 1 and 4 pounds nitrogen per 1,000 square feet.
Brown patch (caused by various Rhizoctonia and Rhizoctonia-like species) is a major summer disease for several cool-season turfgrass species, including creeping bentgrass (Agrostis stolonifera; CBG), colonial bentgrass (Agrostis capillaris; CLBG), perennial ryegrass (Lolium perenne; PRG) and turf-type tall fescue (Schedonorus arundinaceus; TTTF). This destructive summer disease can impact seasonal appearance, uniformity, density and green color, potentially requiring reseeding (2, 5). Preventive fungicide applications can effectively suppress this disease, but implementing cultural management practices such as planting a resistant host or adapting optimized fertilization programs could reduce the overall need for fungicide applications (10, 11, 13).
Among brown-patch-susceptible cool-season turfgrass species, differences in resistance among cultivars within a given species have previously been evaluated. Although differences in cultivar resistance within each species has been previously documented, no studies have directly compared brown patch susceptibility among species grown side by side under identical environmental conditions. In addition to host resistance, other cultural management practices such as nitrogen (N) fertilization practices can affect disease severity. The relationship of brown patch and nitrogen remains complex, but, historically, brown patch incidence and severity have increased as the turfgrass receives higher N rates (1, 4, 6). Currently, best management practices for N fertility in regard to management of brown patch among all cool-season species is to avoid an abundance of quick-release N sources and prevent insufficient N to avoid enhancing disease severity (14).
While previous research has focused on differences among cool-season turfgrass species (e.g., supplemental irrigation needs, N fertilization requirements), relative resistance levels to R. solani among these species remains limited (12, 15). Thus, a controlled-environment study was conducted with the objectives of 1) comparing brown patch severity for four cool-season turfgrass species (CBG, CLBG, PRG and TTTF) and cultivars under identical environmental conditions with the same pathogen isolate when fertilized at two N rates, and 2) determining if differential N rates and subsequent canopy growth rate within each turfgrass species affects brown patch severity.
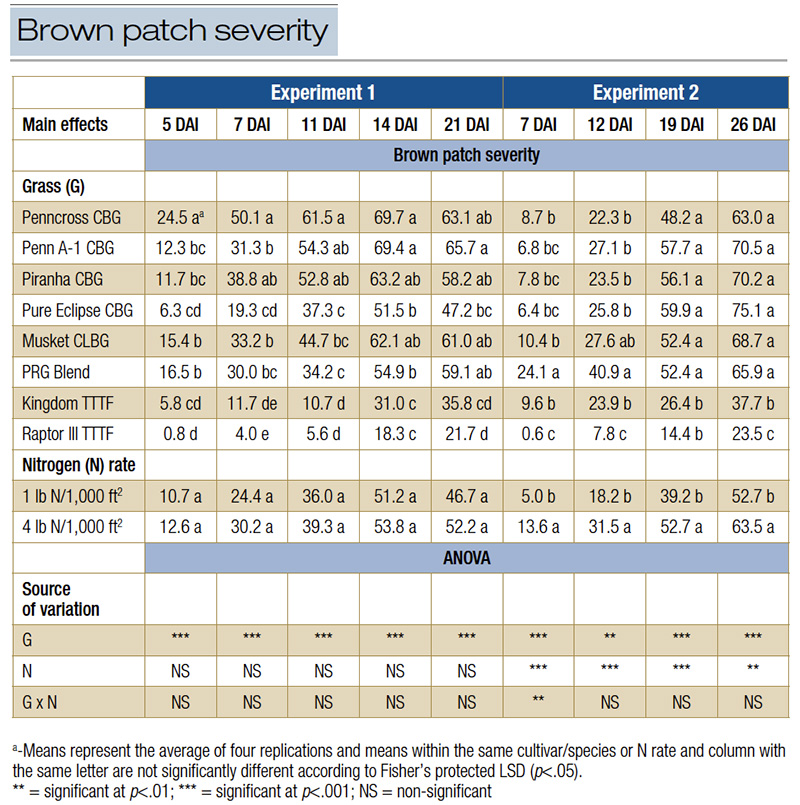
Table 1. Brown patch severity across various days after inoculation (DAI) for creeping bentgrass (CBG), colonial bentgrass (CLBG), perennial ryegrass (PRG) and turf-type tall fescue (TTTF) cultivars as affected by two nitrogen rates during two experiments conducted in a high-humidity, enclosed chamber located inside a controlled environment facility in West Lafayette, Ind.
Materials and methods
A controlled environment study was conducted at the Purdue University Horticulture Plant Growth Facility (West Lafayette, Ind.). Plant samples (greater than 2 years old; 4 square inches or 26 square centimeters) of four CBG cultivars (Penncross, Penn A-1, Piranha and Pure Eclipse), Musket CLBG, a PRG blend (Champion GQ blend) and two TTTF cultivars (Kingdom and Raptor III) were collected from the William H. Daniel Turfgrass Research and Diagnostic Center (Purdue University). The samples were transferred to the controlled-environment facility and established in 4-inch (10.2-centimeter) diameter by 3-inch (7.62-centimeter) deep containers and backfilled with medium-coarse calcareous sand: silt-loam. Weekly N fertilization was initiated on Dec. 23, 2021, (Experiment 1) and March 14, 2022, (Experiment 2) using urea, delivering 0.25 or 1.0 pound of nitrogen per 1,000 square feet (12.21 or 48.82 kilograms per hectare) per week over four consecutive weeks, totaling 1.0 or 4.0 pounds nitrogen per 1,000 square feet (48.82 or 195.3 kilograms per hectare). Turfgrass was hand trimmed with scissors three times per week at 0.5 inch (1.27 centimeters) with clippings removed. Canopy growth rate of each grass was determined by dry matter yield (DMY). One day prior to inoculation, clippings were collected from four days of growth and immediately placed in a drying oven for 48 hours and then weighed.
Turfgrass was inoculated with R. solani during Experiment 1 and 2 on Feb. 7, 2022 (24 days after last N application), and April 25, 2022 (21 days after last N application), respectively. Cultivars were immediately placed in an enclosed chamber covered by translucent plastic that maintained high relative humidity and elevated mean daily temperatures and provided a long leaf wetness duration to promote infection (7). Elevated leaf wetness was achieved using timed misters. Disease severity was determined quantitatively using digital image techniques over a 21- or 26-day period during Experiments 1 and 2, respectively (9). Example images 26 days after inoculation (DAI) during Experiment 2 are provided in Figure 1. Area under the disease progress curve (AUDPC) was calculated to evaluate brown patch severity throughout the experiment using current and previous brown patch severity values and time between measurements.
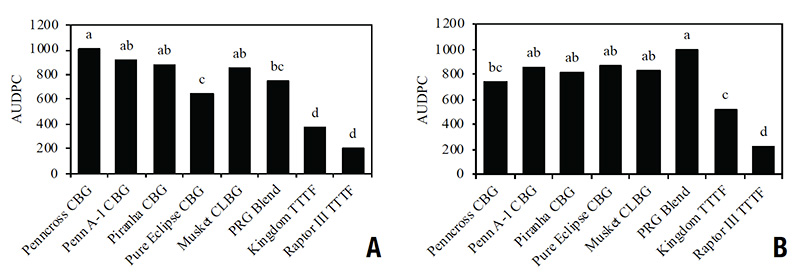
Figure 2. Brown patch area under the disease progress curve (AUDPC) for four cool-season species — creeping bentgrass (CBG), colonial bentgrass (CLBG), perennial ryegrass (PRG) and turf-type tall fescue (TTTF) — and cultivars inoculated with Rhizoctonia solani on Feb. 7, 2022, during Experiment 1 (A) and April 25, 2022, during Experiment 2 (B) after being placed in a high-humidity, enclosed chamber located inside a controlled environment facility in West Lafayette, Ind. Columns within experiment followed by a common letter are not significantly different according to Fisher’s protected LSD (p<.05). Data in columns represent means averaged across two nitrogen rates (1 and 4 pounds nitrogen per 1,000 square feet) and four replications (n=8).
Results
Turf fertilized with 4.0 pounds nitrogen per 1,000 square feet had the greatest canopy growth rate during both experiments, increasing growth by 33% in Experiment 1 and 50% in Experiment 2. Among the cultivars and species, differences were not present when fertilized with 1.0 pound nitrogen per 1,000 square feet, but Piranha CBG, Penn A-1 CBG and Musket CLBG had greater canopy growth compared to Kingdom TTTF when fertilized with 4.0 pounds nitrogen per 1,000 square feet.
In Experiment 1, peak disease (21 DAI) among all species/cultivars ranged between 21.7% and 65.7% for Raptor III TTTF and Penn A-1 CBG, respectively (Table 1). Additionally, differences in brown patch severity between the two N rates among evaluation dates in Experiment 1 were not observed. During Experiment 2, mean brown patch severity at peak disease (26 DAI) ranged from 21.5% to 73.4% for Raptor III TTTF and Pure Eclipse CBG, respectively. Further, the higher N rate increased brown patch severity from 12 DAI to the remainder of the study during Experiment 2 when averaged among the eight cool-season grasses.
The TTTF cultivars reduced brown patch AUDPC by 65.6% compared to CBG, CLBG and PRG during Experiment 1 (Figure 2A). Among the CBG cultivars, Pure Eclipse reduced overall brown patch by 36.6% compared to Penncross, Penn A-1 and Piranha. During Experiment 2, Kingdom and Raptor III TTTF reduced brown patch AUDPC by 38.0% and 75.5%, respectively, compared to CBG, CLBG and PRG (Figure 2B). For the TTTF cultivars, Raptor III had reduced brown patch AUDPC by 56.7% compared to Kingdom. Differences in brown patch AUDPC among the CBG cultivars were not observed. Across all cultivars, AUDPC increased with the higher N rate compared to the lower N rate (797.7 versus 634.8, respectively).
Brown patch AUDPC between N rates was also independently analyzed within each cool-season species (CBG and TTTF cultivars averaged). For CBG, the higher N rate increased brown patch AUDPC by 17% compared to the lower N rate (915.9 versus 762.7, respectively). Brown patch AUDPC also increased by 33% for the higher N rate compared to the lower N rate for the PRG blend (1,046.2 versus 696.6, respectively). When comparing DMY to brown patch AUDPC within individual species using linear regression, a positive relationship was observed for CBG cultivars and the PRG blend (i.e., brown patch severity increased as DMY increased) (Figure 3). There were no differences in brown patch AUDPC between N rates for CLBG or TTTF, and a relationship between DMY and AUDPC for these species was not observed.
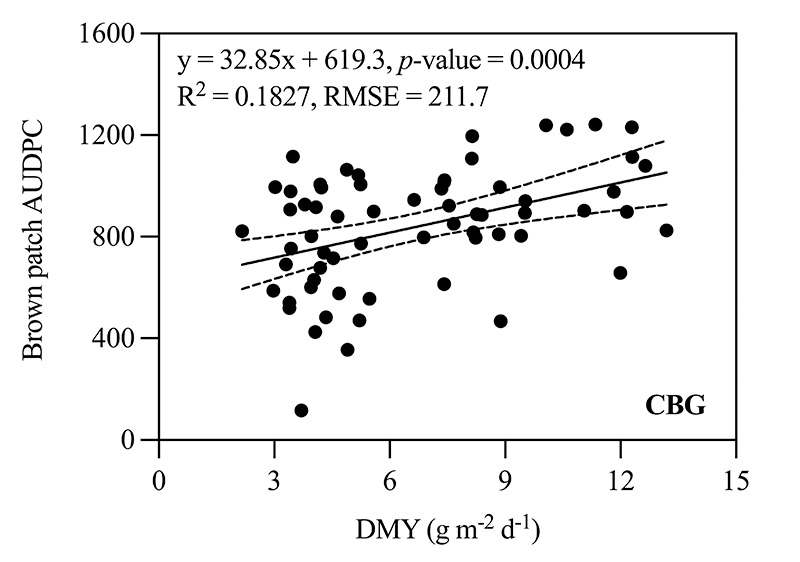
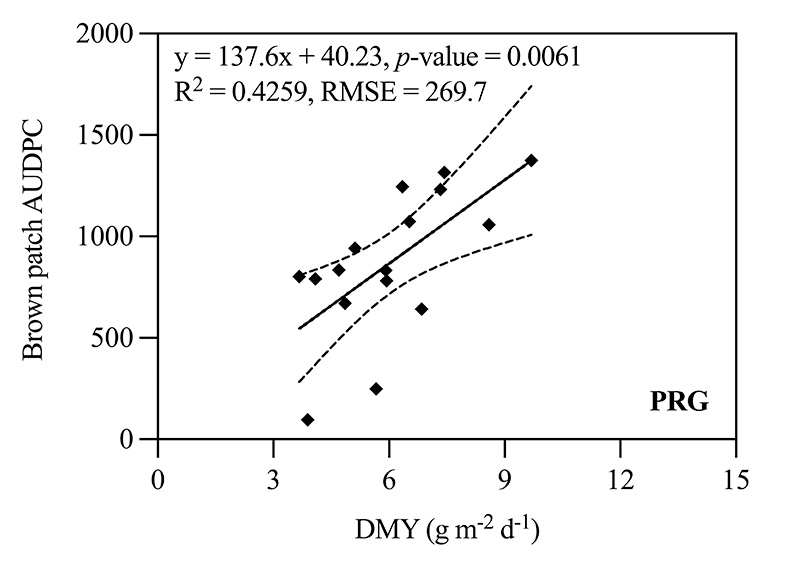
Figure 3. Relationship of relative growth rate (DMY) and brown patch area under the disease progress curve (AUDPC) for four creeping bentgrass (CBG) cultivars (Penncross, Penn A-1, Piranha and Pure Eclipse) and a perennial ryegrass (PRG) blend (Champion GQ blend) as affected by two nitrogen (N) rates totaling 1 and 4 pounds nitrogen per 1,000 square feet when inoculated in an enclosed chamber located inside a controlled environment facility in West Lafayette, Ind. Dashed lines represent 95% confidence intervals.
Conclusion
Through turfgrass breeding efforts, improvements in cultivar disease resistance across cool-season turfgrass species has allowed for host resistance to be utilized as an effective disease management strategy (10). Results from this study demonstrate that certain turfgrass species, such as TTTF, have increased brown patch resistance compared to other cool-season species (CBG, CLBG and PRG), and the influence of N rate on brown patch severity is species dependent. These results reinforce the importance of planting disease-resistant species and cultivars. Further, traditional management recommendations have been to limit or avoid N applications during the spring and summer to avoid enhancing brown patch severity across all cool-season turfgrass species (5, 14). In this controlled environment study, cultivar resistance was more of a significant factor than N rate. Additionally, due to brown patch AUDPC increasing as DMY increased for PRG and CBG, management practices that promote increased rates of DMY during environmental conditions favorable for brown patch development should be limited for these species based on the results from this study.
Overall, this study reinforces the importance of planting resistant cultivars and species in locations where environmental conditions are favorable for brown patch. The role of N and the relationship between specific pathogens and hosts should be further evaluated under field conditions across multiple environments based on the results of this controlled environment study. Further, supplemental N application rates among the species evaluated should be based on the environmental location, turfgrass species, soil conditions, maintenance goals, visual expectations and time of the year. Although the higher N rates used in this study did not enhance overall brown patch (AUDPC) for TTTF or CLBG, high N rates should be avoided due to the potential excess DMY, frequent mowing requirements and potential off-target nutrient movement (3, 8). Future recommendations for best management practices to minimize brown patch, especially for N recommendations, should be based on individual species rather than equating similar responses in N rate and brown patch across all turfgrass species.
The research says
- Certain turfgrass species such as turf-type tall fescue have increased brown patch resistance compared to other cool-season species (creeping bentgrass, colonial bentgrass and perennial ryegrass), and the influence of nitrogen rate on brown patch severity is species dependent.
- In this controlled environment study, cultivar resistance was more of a significant factor than nitrogen rate as a means to avoid enhancing brown patch severity across all cool-season turfgrass species.
- Management practices that promote excessive rates of dry matter yield during environmental conditions favorable for brown patch development should be limited for these species based on the results from this study.
Acknowledgements
Funding for this research was provided by the Midwest Regional Turfgrass Foundation (West Lafayette, Ind.) and the Purdue College of Agriculture (West Lafayette, Ind.). The authors would like to thank Emma Seward and Max Schimmel for their assistance in maintaining this study.
This article is intended for educational purposes. Further comprehensive information can be accessed in our original publication in Crop Science (https://doi.org/10.1002/csc2.21256).
Literature cited
- Bloom, J.R., and H.B. Couch. 1960. Effect of nutrition, pH, and soil moisture on Rhizoctonia brown patch. Phytopathology 50:532-535.
- Burpee, L., and B. Martin. 1992. Biology of Rhizoctonia species associated with turfgrass. Plant Disease 76:112-117.
- Burwell Jr., R.W., J.S. Beasley, L.A. Gaston, S.M. Borst, R.E. Sheffield, R.E. Strahan and G.C. Munshaw. 2011. Losses of surface runoff, total solids, and nitrogen during bermudagrass establishment on levee embankments. Journal of Environmental Quality 40(4):1241-1248 (https://doi.org/10.2134/jeq2010.0458).
- Butler, E.L., G.H. Galle and J.P. Kerns. 2019. Influence of nitrogen rate and timing, fungicide application method, and simulated rainfall after fungicide application on brown patch severity in tall fescue. Crop, Forage and Turfgrass Management 5(1):1-6 (https://doi.org/10.2134/cftm2019.03.0018).
- Couch, H.B. 1995. Diseases of turfgrasses. Third edition. Krieger Publishing, Malabar, Fla.
- Fidanza, M.A., and P.H. Dernoeden. 1996. Brown patch severity in perennial ryegrass as influenced by irrigation, fungicide, and fertilizers. Crop Science 36(6):1631-1638 (https://doi.org/10.2135/cropsci1996.0011183X003600060036x).
- Fidanza, M.A., P.H. Dernoeden and A.P. Grybauskas. 1996. Development and field validation of a brown patch warning model for perennial ryegrass turf. Phytopathology 86:385-390.
- Frank, K.W., K.M. O’Reilly, J.R. Crum and R.N. Calhoun. 2006. The fate of nitrogen applied to a mature Kentucky bluegrass turf. Crop Science 46(1):209-215. (https://doi.org/10.2135/cropsci2005.04-0039).
- Karcher, D.E., and M.D. Richardson. 2013. Digital image analysis in turfgrass research. In: J.C. Stier, B.P. Horgan and S.A. Bonos, eds. Turfgrass: Biology, Use, and Management. (Agronomy Monograph 56:1133-1149). ASA, CSSA and SSSA., Madison, Wis.
- Kerns, J. 2023. Advances in turfgrass disease management. In: M. Fidanza, ed. Achieving sustainable turfgrass management. Burleigh Dodds Science Publishing, Cambridge, England.
- Koch, P.L., and J.P. Kerns. 2012. Relative resistance of creeping bentgrass cultivars to Sclerotinia homoeocarpa and Typhula incarnata. Applied Turfgrass Science 9(1):1-5 (https://doi.org/10.1094/ATS-2012-1022-01-RS).
- Powlen, J.S., and C.A. Bigelow. 2023. Cool-season golf course fairway species irrigation requirements under limited irrigation. Crop, Forage & Turfgrass Management 9(1):e20205 (https://doi.org/10.1002/cft2.20205).
- Settle, D., J. Fry and N. Tisserat. 2001. Dollar spot and brown patch fungicide management strategies in four creeping bentgrass cultivars. Crop Science 41(4):1190-1197 (https://doi.org/10.2135/cropsci2001.4141190x).
- Tredway, L.P., M. Tomaso-Peterson, J.P. Kerns and B.B. Clarke. 2023. Compendium of Turfgrass Diseases. Fourth edition. American Phytopathological Society, St. Paul, Minn.
- Walker, K.S., C.A. Bigelow, D.R. Smith, G.E. Van Scoyoc and Z.J. Reicher. 2007. Aboveground responses of cool-season lawn species to nitrogen rates and application timings. Crop Science 47(3):1225-1236 (https://doi.org/10.2135/cropsci2006.09.0595).
Jada Powlen (jpowlen@purdue.edu) is a lead research scholar, and Cale Bigelow, Ph.D., is a professor of turf science, management and ecology in the Department of Horticulture and Landscape Architecture at Purdue University, West Lafayette, Ind. Jim Kerns is a professor and Extension specialist of turfgrass pathology in the Department of Entomology and Plant Pathology at North Carolina State University in Raleigh, N.C. Mike Fidanza is a professor of plant and soil sciences at Pennsylvania State University, Berks Campus in Reading, Pa.