
Figure 1. For this research, treatments were applied to replicated field plots on Pennlinks creeping bentgrass maintained as a putting green. Photos by Trevor Stacy
Editor’s note: Browse all fungicide-related research and articles recently published in GCM.
Given that most fungicides are mixed with water and applied as dilute sprays, it is reasonable to consider water quality as a factor that affects fungicide performance. Water quality is defined in terms of three factors: pH (a measure of acidity or alkalinity of an aqueous solution), hardness (measure of the amount of positively charged ions — calcium, magnesium and iron — in water) and turbidity (measure of the relative clarity of a liquid). Hardness and pH are known to alter the effectiveness of some organophosphate insecticides and select groups of herbicides (2). There is also ample evidence demonstrating the effects of water pH and hardness on the performance of plant growth regulators (PGRs) and herbicides used to manage turfgrass (4).
As far as fungicides are concerned, only the pH factor has been implicated in affecting product performance. The concern is over alkaline hydrolysis, a process whereby active ingredients are degraded into metabolites that are not toxic to fungi, in carrier water with pH greater than 7.0.
Interestingly, the body of published research regarding pH effects on fungicide efficacy and disease control is not robust and is mostly limited to a few single-season fungicide trials and scant data from studies with crop pathogens. Despite the absence of detailed research on the pH-fungicide issue, the general consensus since the early 2000s has been that water quality (alkaline carrier water pH) contributes to unsatisfactory disease control.
Testing the effects of pH on three fungicides
Our objective, as part of broader research on factors affecting fungicide performance, was to investigate the influence of water pH on the efficacy of three fungicides applied for dollar spot control on creeping bentgrass: 26GT (iprodione, Bayer); Tourney (metconazole, Nufarm); and 3336 F (thiophanate-methyl, Nufarm) (3).
For the field experiments, we prepared fungicide suspensions in carrier water with pH fixed at three levels: 5.0, 7.0 and 9.0. A time factor was also included: For time T0, fungicides were applied immediately upon mixing; and for time T24, fungicides were mixed and kept under constant agitation for 24 hours before application. For each of four runs of the experiment, fungicide suspensions were applied once to replicated field plots (3.3 feet × 6.6 feet; 1 × 2 meters), and then disease severity was recorded at three- to four-day intervals for four weeks (Figure 1). Area under the disease progress curve (AUDPC) was calculated to represent a measure of disease severity over the course of each experimental run.
The field research sites were swards of Pennlinks or Crenshaw creeping bentgrass maintained as a putting green, mowed six times per week at a height of 0.16 inch (4 millimeters) and fertilized at approximately 2.5 pounds nitrogen/1,000 square feet/year (122 kilograms/hectare/year). Three runs were completed during spring and summer 2016, and a fourth run was completed during summer 2017. Three runs on Pennlinks were initiated before symptoms appeared in plots. Sprays on Crenshaw were initiated post-outbreak — that is, symptoms were already evident when fungicide was applied.
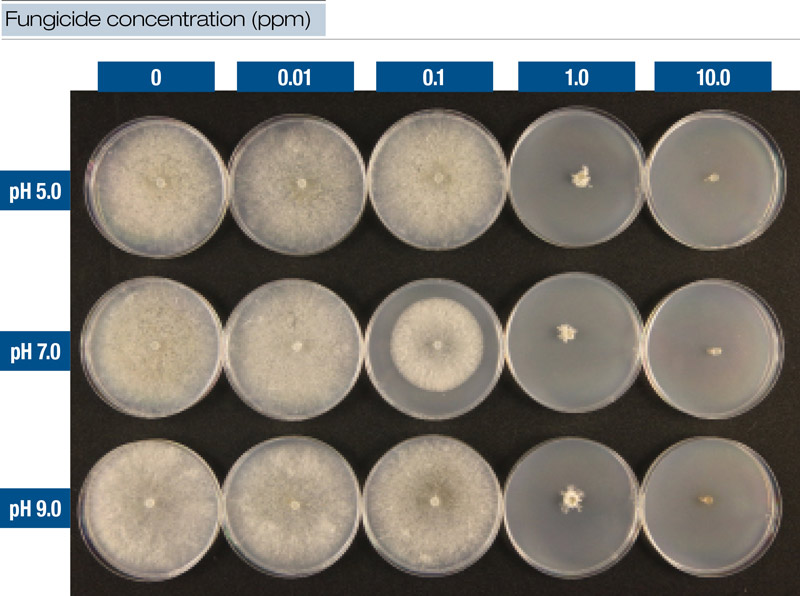
Figure 2. Fungicide was mixed with water at three pH levels and added to culture medium at five dilutions. This figure illustrates results from iprodione at time T0 with Clarireedia species isolate PU1616.
Laboratory experiments involved mixing fungicides with water at each pH level and then adding mixtures to culture media (potato dextrose agar, PDA) to create a series of five dilutions (0.0, 0.01, 0.1, 1.0 and 10.0 ppm) (Figure 2, above). The time factor was also included in the laboratory experiments so that for T0, culture plate dilutions were created immediately after mixing. For T24, the dilution plates were prepared 24 hours after fungicides were mixed with water at each pH level. Locally collected isolates (designated PU1616 and PU0809) of Clarireedia species were transferred to culture plates and incubated for 72 hours. After incubation, colony diameters were measured and recorded.
We used relative growth (colony growth of each dilution relative to colony growth on PDA with no fungicide) to calculate the effective concentration at which colony growth on fungicide-amended culture plates was reduced to 50% of colony growth on culture plates with no fungicide (EC50). AUDPC data from field studies and EC50 values from laboratory experiments were subjected to statistical analysis.
Results
Field results
Statistical analysis revealed that carrier water pH had no effect on the efficacy of metconazole and thiophanate-methyl for controlling dollar spot. Regardless of whether the treatments were applied immediately after mixing or agitated and delayed for 24 hours, no statistical difference in disease severity (AUDPC) was identified for all four runs. For iprodione treatments, the only significant difference involved carrier water at pH = 9.0 and time factor T24. In that instance, AUDPC (1,196.5) was significantly greater than in all other iprodione treatments, but in only one of four runs (Table 1).
Lab results
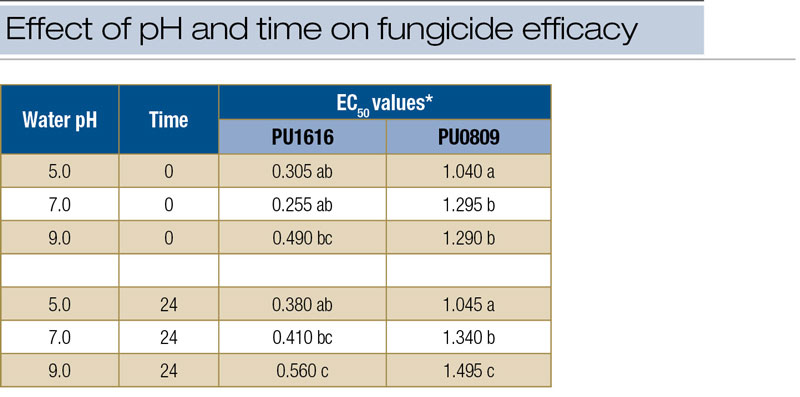
* For each isolate, EC50 values followed by the same letter are not significantly different.
Table 2. EC50 values for two isolates (PU1616 and PU0809) of Clarireedia species growing on culture media amended with iprodione mixed in water at pH 5.0, 7.0 and 9.0. At time T0, fungicides were added to media immediately after mixing. For time T24, fungicide was added to media 24 hours after mixing.
Statistical analysis of EC50 values generated in laboratory fungicide assays showed significant pH effects for iprodione and significant time factor effects for iprodione and metconazole. Results were consistent for both isolates of the pathogen included in the assay. No significant effects for carrier water pH and/or time factor were identified for thiophanate-methyl. Comparison of treatment means for iprodione revealed that for pH 9.0 and T24, EC50 values (for both isolates) were higher than those for all other treatment means (Table 2, above). No differences were identified among mean EC50 values for thiophanate-methyl. For metconazole, significant differences among treatments occurred but revealed only that EC50 values were lower at T0 than at T24 (data not shown).
Conclusions and interpretations
Many factors, including water quality, contribute to the effectiveness of products applied to maintain turf health. Results of this research established that pH of spray carrier water had some effect on efficacy of iprodione and no effect on efficacy of thiophanate-methyl and metconazole against dollar spot on creeping bentgrass. They contribute to the interpretation that, with perhaps few exceptions, modern fungicide formulations are not likely to lose efficacy as a result of carrier water pH (3).
The research was limited to the specific issue of alkaline hydrolysis and fungicide efficacy. From a practical perspective, the likelihood that alkaline hydrolysis will affect fungicide performance is remote for several reasons. The first involves the characteristic low solubility of these fungicides (Table 3, below) and fungicides in general (1). The amount of active ingredient subject to hydrolytic breakdown is a function of solubility in water. For fungicides with low solubility, only small amounts of active ingredient are vulnerable to breakdown at any given time. Second, fungicides rarely remain in the tank for more than a few hours, limiting the time for the degradation process. Third, inert ingredients in the fungicide formulation invariably contain a variety of stabilizing agents that buffer against breakdown of active ingredients in highly alkaline water.
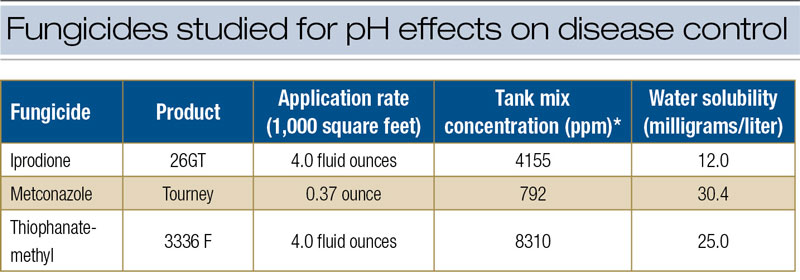
* Concentration based on spray volume: 1.75 gallons/1,000 square feet.
** Fungicide concentration required for 50% reduction in colony growth on culture media with no fungicide.
Table 3. Characteristics of fungicides studied for pH effects on disease control.
Finally, the labeled application rates are high relative to fungicide concentrations required to slow or stop fungal growth. In culture plates, growth of Clarireedia species is reduced at fungicide concentrations ranging from 0.1 to 1.0 ppm, but concentrations in the spray tank are orders of magnitude greater. For example, the culture plate experiments showed that the EC50 for iprodione (against Clarireedia species) ranged from 0.26 to 0.56 ppm for isolate PU1616 and from 1.04 to 1.34 ppm for isolate PU0809 (Table 2). However, in the field research, the equivalent of 4.0 fluid ounces of 26GT in 1.75 gallons water/1,000 square feet (713 liters/hectare) represented a concentration of 4,155 ppm in the spray tank (Table 3).
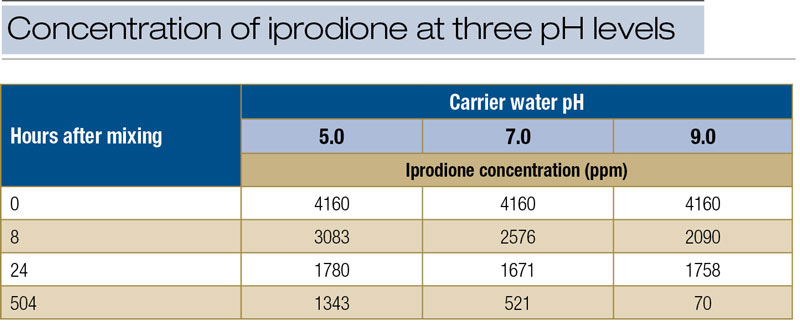
Table 4. Concentration of iprodione (ppm) detected in fungicide suspensions mixed with water at three pH levels.
Our study also included a quantitative determination of the breakdown of iprodione over time in water stabilized at the three pH levels. The results showed that, for iprodione, an initial fungicide concentration of 4,160 ppm was reduced to 74.1%, 61.9% and 50.2% after eight hours at pH 5.0, 7.0 and 9.0, respectively (Table 4, above). Therefore, although the half-life of iprodione in an aqueous solution at pH 9.0 is listed as 0.02 of a day (less than 30 minutes), half of the initial concentration still remained after eight hours because only the soluble portion (12 ppm) is vulnerable to breakdown at any given time. Furthermore, at 504 hours (21 days after mixing), concentration of iprodione in water ranged from 1,343 to 70 ppm, depending on pH (Table 4). The practical interpretation is that, in the normal course of applying fungicides to turf, the likelihood that alkaline hydrolysis will adversely affect product performance is very low and near zero for modern fungicides.
Alkaline hydrolysis is separate from compatibility of tank-mix partners. The need for buffering agents to facilitate compatibility increases with the number and variety of components added to the tank. So, there are practical reasons for stabilizing pH, but concern over unsatisfactory fungicide performance caused by alkaline hydrolysis is not among them.
The research says ...
- A small group of research papers has suggested that the pH of the carrier water could reduce the efficacy of fungicides.
- Lab and field tests were used to test the effects of carrier water pH on three widely used fungicides: iprodione, metconazole and thiophanate-ethyl.
- The likelihood that alkaline hydrolysis will cause unsatisfactory performance is near zero for modern fungicides.
Editor’s note: Browse all fungicide-related research and articles recently published in GCM.
Literature cited
- Latin, R. 2020. A practical guide to turfgrass fungicides. Second edition. APS Press, St. Paul, Minn. In press.
- Sarmah, A.K., R.S. Kookana, M.J. Duffy, A.M. Alston and B.D. Harch. 2000. Hydrolysis of triasulfuron, metsulfuron-methyl and chlorsulfuron in alkaline soil and aqueous solutions. Pest Management Science 56:463-471. (https://doi.org/10.1002/(SICI)1526-4998(200005)56:5<463::AID-PS138>3.0.CO;2-I).
- Stacy, T., and R. Latin. 2020. The influence of water pH on efficacy fungicides for turf disease control. Crop, Forage & Turfgrass Management. (https://doi.org/10.1002/cft2.20007).
- Thelen, K.D., E.P. Jackson and D. Penner. 1995. The basis for the hard-water antagonism of glyphosate activity. Weed Science 43:541-548. (https://doi.org/10.1017/S0043174500081613).
Rick Latin, professor emeritus from Purdue University and 2019 recipient of GCSAA’s Distinguished Service Award, is currently a consultant working in Pinehurst, N.C. Trevor Stacy was a graduate research assistant at Purdue University when this work was done and is now an associate investigator in FMC’s fungicide discovery group.